Week-6 - OSU Chemistry
advertisement

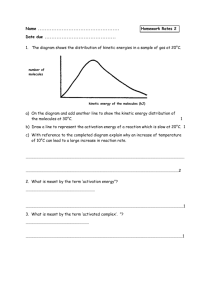
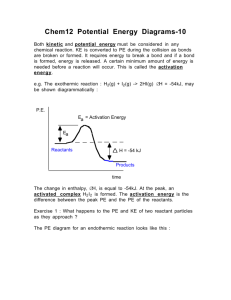
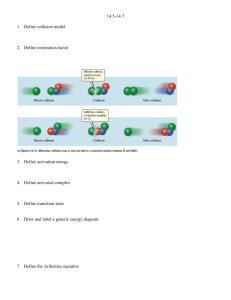
Quiz # 4 (Feb 14-18) will be on sections 14.5-14.7, and will be given in recitation Take out a clean sheet of paper and print on it your name and section number and an indication it is “pop quiz #1”. Mon Lab, 2:30-5:18 Wed Lab, 2:30-5:18 Pat Bullinger Chris Beekman Edwin Motari Hai Liu Lin Sun Chitanya Patwardhan Jeremy White 96 Roxana Sierra 97 Ramesh Sharma 98 Lin Sun 99 Mark Lobas 100 Chitanya Patwardhan 101 90 91 92 93 94 95 Fri: Patric Benziher 102 Hai Liu 103 Take out a clean sheet of paper and print on it your name and section number and an indication it is “pop quiz #1”. Wed Lab, 6:30-9:18 Fri Lab, 11:30-2:18 Jen Kljun Namrata Singh Mike Johansen Ray Chammas Travis Steinke Lisa Park Sara Wicke Patrick Veres 104 105 106 107 108 109 110 111 A certain reaction has the form A B. At 400 K and [A]0 = 2.8 x 10 -3 M, data for a plot of [A] vs t were collected. It was then found that a plot of 1/[A] vs t yielded a straight line with a slope of 3.60 x 10-2 L∙mol -1 ∙s -1 . a) Write an expression for the rate law. rate = k[A]2 b) Write an expression for the integrated rate law. 1/[A] = 1/[A]0 + kt c) What is the rate constant for the reaction? 3.60 x 10-2 L∙mol -1 ∙s -1 d) What is the half-life for the reaction, as given? 1 1 10 5 3 t1 9 . 9 x 10 2 k [ A]0 (3.60 x102 )( 2.8 x10 3 ) (3.6)( 2.8) A certain reaction has the form A B. At 400 K and [A]0 = 5.6 x 10 -3 M, data for a plot of [A] vs t were collected. It was then found that a plot of 1/[A] vs t yielded a straight line with a slope of 1.80 x 10-2 L∙mol -1 ∙s -1 . a) Write an expression for the rate law. rate = k[A]2 b) Write an expression for the integrated rate law. 1/[A] = 1/[A]0 + kt c) What is the rate constant for the reaction? 1.80 x 10-2 L∙mol -1 ∙s -1 d) What is the half-life for the reaction, as given? 1 1 10 5 3 t1 9 . 9 x 10 2 k [ A]0 (1.80 x102 )(5.6 x10 3 ) (1.8)(5.6) Week six, a continuation of Chapter 14 Chemical Kinetics 14.1 14.2 14.3 Factors that Affect Reaction Rates Reaction Rates Concentration and Rate 14.4 The Change of Concentration with Time 14.5 Temperature and Rate The Collision Model Activation Energy The Orientation Factor The Arrhenius Equation and Activation Energies Reaction Mechanisms Elementary Steps; Multistep Mechanisms Rate Laws for Elementary Steps Rate Laws for Multistep Mechanisms Catalysis Homogeneous and Heterogeneous Catalysis Enzymes 14.5 14.7 Note the DRAMATIC effect of temperature on k Temperature and Rate The Collision Model eg H2 + I2 The Collision Model • The more molecules present, the greater the probability of collision and the faster the rate. • Complication: not all collisions lead to products. In fact, only a small fraction of collisions lead to product. • The higher the temperature, the more energy available to the molecules and the faster the rate. • In order for reaction to occur the reactant molecules must collide in the correct orientation and with enough energy to form products. Activation Energy • Arrhenius: molecules must posses a minimum amount of energy to react. Why? – In order to form products, bonds must be broken in the reactants. – Bond breakage requires energy. • Activation energy, Ea, is the minimum energy required to initiate a chemical reaction. Activation Energy Activation Energy • Consider the rearrangement of acetonitrile: H3C N C H3C N C H3C C N – In H3C-NC, the C-NC bond bends until the C-N bond breaks and the NC portion is perpendicular to the H3C portion. This structure is called the activated complex or transition state. – The energy required for the above twist and break is the activation energy, Ea. – Once the C-N bond is broken, the NC portion can continue to rotate forming a C-CN bond. Activation Energy • The change in energy for the reaction is the difference in energy between CH3NC and CH3CN. • The activation energy is the difference in energy between reactants, CH3NC and transition state. • The rate depends on Ea. • Notice that if a forward reaction is exothermic (CH3NC CH3CN), then the reverse reaction is endothermic (CH3CN CH3NC). Activation Energy • Consider the reaction between Cl and NOCl: – If the Cl collides with the Cl of NOCl then the products are Cl2 and NO. – If the Cl collided with the O of NOCl then no products are formed. • We need to quantify this effect. Activation Energy The Arrhenius Equation • Arrhenius discovered most reaction-rate data obeyed the Arrhenius equation: k Ae Ea RT – k is the rate constant, Ea is the activation energy, R is the gas constant (8.314 J/K-mol) and T is the temperature in K. – A is called the frequency factor, and is a measure of the probability of a favorable collision. – Both A and Ea are specific to a given reaction. The Arrhenius Equation • If we have a lot of data, we can determine Ea and A graphically by rearranging the Arrhenius equation: Ea ln k ln A RT • If we do not have a lot of data, then we can use k2 Ea 1 1 ln k1 R T2 T1 Sample Exercise 14.8 Temp. /oC k / (s-1 ) 189.7 2.52 x 10-5 198.9 5.25 x 10-5 230.3 6.30 x 10-5 251.2 3.16 x10-5 Sample Exercise 14.8 Temp. (oC) T (K) 1/T (K-1) k (s-1 ) 189.7 2.52 x 10-5 198.9 5.25 x 10-5 230.3 6.30 x 10-5 251.2 3.16 x10-5 ln k Sample Exercise 14.8 Temp. (oC) T (K) 1/T (K-1 x 103) k (s-1 ) 189.7 462.9 2.160 2.52 x 10-5 198.9 472.1 2.118 5.25 x 10-5 230.3 503.5 1.986 6.30 x 10-5 251.2 524.4 1.907 3.16 x10-5 ln k Sample Exercise 14.8 Temp. (oC) T (K) 1/T (K-1 x 103) k (s-1 ) ln k 189.7 462.9 2.160 2.52 x 10-5 -10.589 198.9 472.1 2.118 5.25 x 10-5 -9.855 230.3 503.5 1.986 6.30 x 10-5 -7.370 251.2 524.4 1.907 3.16 x10-5 -5.757 Fig 14.18 For CH3NC CH3CN reaction From the graph we find the slope = -1.9 x 104 K But this is also equal to - Ea/R Or Ea = -(slope)(R) = - ( - 1.9x104 )(8.314 J mol-1 K-1)(1 kJ / 1000 J) = 1.62 x 102 kJ/mol or 162 kJ/mol We can now use these results to calculate the rate constant at any temperature. k2 Ea 1 1 ln k1 R T2 T1 To calculate k1 for a temperature of 430.0 K, make substitutions for all other parameters: k2 = 2.52 x 10-5 s-1 T2 = 462.9 K And T1 = 430.0 K to obtain k1 = 1.0 x 10-6 s -1 Activation Energy-orientation factor k Ae Ea RT Note that termolecular reactions are extremely unlikely ! Multistep Mechanisms and Rate Laws Overall: NO2 (g) + CO (g) NO (g) + CO2 (g) with an observed rate law of Rate = k[NO2]2 It appears that at temperatures below 225 oC, the reaction proceeds via two elementary steps: Which yields two rate expressions: NO2 + NO2 NO3 + NO NO3 + CO NO2 + CO2 Rate1 = k1 [NO2]2 Rate2 = k2 [NO3][CO] (1) (2) Important points: (a) Multi steps must add up to yield overall reaction. (b) may involve reactive intermediates (different from activated complexes). (c) One of these may be the ‘rate-determining’ step—the slower one! In this case given above, and step (1) is the rate-determining step step (2) is a faster step. Another example: 2 NO2 + F2 2 NO2F observed rate = k[NO2][F2] Proposed mechanism: NO2 + F2 NO2F + F F + NO2 NO2F (1) slow (2) fast Sum gives overall reaction, and rate = rate1 = k1[NO2][F2] Somewhat more complicated: 2 O 3 3 O2 obs rate = k[O3]2[O2] -1 Proposed mechanism: O3 == O2 + O (1) fast, reversible O + O3 2 O2 (2) slow tells us rate = k2[O][O3] now what??? assume k1[O3] = k -1[O2][O] so that k1[O3 ] [O3 ] [O] k' k 1[O2 ] [O2 ] !!! k1[O3 ] [O3 ] [O ] k' k 1[O2 ] [O2 ] !!! and rate k2 [O ][O3 ] becomes 2 k1 [O3 ] [O3 ] rate k2 [O3 ] k k 1 [O2 ] [O2 ] Another example: overall with an observed rate law of 2 NO + Br2 2 NOBr rate = k[NO]2[Br2] The proposed mechanism is: NO + Br2 == NOBr2 (1) (fast) NOBr2 + NO NOBr (2) (slow) Our earlier guideline would suggest we use the slow step to determine the rate law, giving rate = k2[NOBr2][NO] but this presents an immediate problem, since we don’t know what experimental quantities to put in for [NOBr2] ! The solution comes from an analysis of the reversible fast reaction (1). rate forward = k 1[NO][Br2] and rate reverse = k -1[NOBr2] but these exist in a fast, dynamic equilibrium where rate forward = rate reverse And this gives us the relationship rate forward = k 1[NO][Br2] = k -1[NOBr2] = rate reverse and [ NOBr2 ] k1 k 1 [ NO][ Br2 ] and finally k1 Rate k2 [ NOBr2 ][ NO] k2 [ NO][ Br2 ][ NO] k1 k [ NO]2 [ Br2 ] Quiz #4 Next Week (Feb 14-17) Covers sections 14.5-14.7 During lectures next week, we will cover ALL OF Chapter 15! The Second Midquarter Exam will cover Chapters 14 and 15. Take out a clean sheet of paper and print on it your name and section number and an indication it is “pop quiz #1a”. Mon Lab, 2:30-5:18 Wed Lab, 2:30-5:18 Pat Bullinger Chris Beekman Edwin Motari Hai Liu Lin Sun Chitanya Patwardhan Jeremy White 96 Roxana Sierra 97 Ramesh Sharma 98 Lin Sun 99 Mark Lobas 100 Chitanya Patwardhan 101 90 91 92 93 94 95 Fri: Patric Benziher 102 Hai Liu 103 A certain reaction has the form A B. At 400 K and [A]0 = 2.8 x 10 -3 M, data for a plot of [A] vs t were collected. It was then found that a plot of 1/[A] vs t yielded a straight line with a slope of 3.60 x 10-2 L∙mol -1 ∙s -1 . a) Write an expression for the rate law. rate = k[A]2 b) Write an expression for the integrated rate law. 1/[A] = 1/[A]0 + kt c) What is the rate constant for the reaction? 3.60 x 10-2 L∙mol -1 ∙s -1 d) What is the half-life for the reaction, as given? 1 1 10 5 3 t1 9 . 9 x 10 2 k [ A]0 (3.60 x102 )( 2.8 x10 3 ) (3.6)( 2.8) Cl2 + CHCl3 HCl + CCl4 rate = k[Cl2]1/2[CHCl3] Proposed mechanism: Cl2 == 2 Cl (1) fast, reversible Cl + CHCl3 HCl + CCl3 (2) slow CCl3 + Cl CCl4 (3) fast Evaluate the rate constant using this mechanism. Catalysis A catalyst changes the rate of a chemical reaction without being consumed. • Homogeneous catalysis: catalyst and reaction are in the same, single phase. • Heterogeneous catalysis: catalyst and reaction are in different phases. Often the catalyst is a solid in contact with gaseous or liquid reactions. • Enzymes: In living systems, usually a large molecule which catalyzes a specific reaction, an enzyme-substrate complex. • Homogeneous: catalyst and reaction are in same phase: • Hydrogen peroxide decomposes very slowly: 2H2O2(aq) 2H2O(l) + O2(g) • In the presence of the bromide ion, the decomposition occurs rapidly: – – – – 2Br-(aq) + H2O2(aq) + 2H+(aq) Br2(aq) + 2H2O(l). Br2(aq) is brown. Br2(aq) + H2O2(aq) 2Br-(aq) + 2H+(aq) + O2(g). Br- is a catalyst because it can be recovered at the end of the reaction and it makes the reaction rate faster. • Generally, catalysts operate by lowering the activation energy for a reaction. • Catalysts can operate by increasing the number of effective collisions. • That is, from the Arrhenius equation: catalysts increase k be increasing A or decreasing Ea. • A catalyst may add intermediates to the reaction. • Example: In the presence of Br-, Br2(aq) is generated as an intermediate in the decomposition of H2O2. • When a catalyst adds an intermediate, the activation energies for both steps must be lower than the activation energy for the uncatalyzed reaction. • • • • • • Heterogeneous Catalysis The catalyst is in a different phase than the reactants and products. Typical example: solid catalyst, gaseous reactants and products (catalytic converters in cars). Most industrial catalysts are heterogeneous. First step is adsorption (the binding of reactant molecules to the catalyst surface). Adsorbed species (atoms or ions) may have increased reactivity, but they are always easily available. Reactant molecules are also adsorbed onto the catalyst surface and may migrate to active sites. – Consider the hydrogenation of ethylene: C2H4(g) + H2(g) C2H6(g), H = -136 kJ/mol. – The reaction is slow in the absence of a catalyst. – In the presence of a metal catalyst (Ni, Pt or Pd) the reaction occurs quickly at room temperature. – First the ethylene and hydrogen molecules are adsorbed onto active sites on the metal surface. – The H-H bond breaks and the H atoms migrate about the metal surface. – When an H atom collides with an ethylene molecule on the surface, the C-C bond breaks and a C-H bond forms. – When C2H6 forms it desorbs from the surface. – When ethylene and hydrogen are adsorbed onto a surface, less energy is required to break the bonds and the activation energy for the reaction is lowered. • • • • • • • ENZYMES Enzymes are biological catalysts. Most enzymes are protein molecules with large molecular masses (10,000 to 106 ). Enzymes have very specific shapes. Most enzymes catalyze very specific reactions. Substrates are the reactants that undergo reaction at the active site of an enzyme. A substrate locks into an enzyme and a fast reaction occurs. The products then move away from the enzyme. Considerable research is currently underway to modify enzymes to prevent undesirable reactions and/or to prepare new products. • Only substrates that fit into the enzyme lock can be involved in the reaction. • If a molecule binds tightly to an enzyme so that another substrate cannot displace it, then the active site is blocked and the catalyst is inhibited (enzyme inhibitors). • The number of events (turnover number) catalyzed is large for enzymes (103 - 107 per second). ‘Fixation’ of N2’ converts it to compounds useful to plants Nitrogenase in legumes converts N2 to NH3. Consider the reaction 2 N2O5 4 NO2 + O2 Calculate Ea from the following data: k/s-1 2.0 x 10-5 7.3 x 10-5 2.7 x 10-4 9.1 x 10-4 2.9 x 10-4 T/oC 20 30 40 50 60 Sample Problem, page 562-563: Formic acid alone, in the gas phase. Formic acid in presence of ZnO. The decomposition of formic acid shown on the previous slide is given by HCOOH (g ) CO2 (g) + H2 (g) It has been found to be first order at 838 K. a) Estimate the half-life and first-order rate constant for the decomposition of pure formic acid and formic acid in the presence of ZnO. b) What is the effect of ZnO? c) Suppose we express the concentration of formic acid in mol/L. What effect would that have on the rate constant? d) The pressure of formic acid at the beginning of the reaction can be read from the graph. Assume constant T, ideal gas behavior, and areaction volume of 436 cm3. How many moles of gas are in the container at the end of the reaction? e) The standard heat of formation of formic acid vapor is ΔHof = -378.6 kJ/mol. Calculate ΔHo for the overall reaction. Assume the activation energy, Ea , is 184 kJ/mol, sketch an approximate energy profile for the reaction, and label Ea, ΔHof , and the transition state.