Acid/Base Titration
advertisement
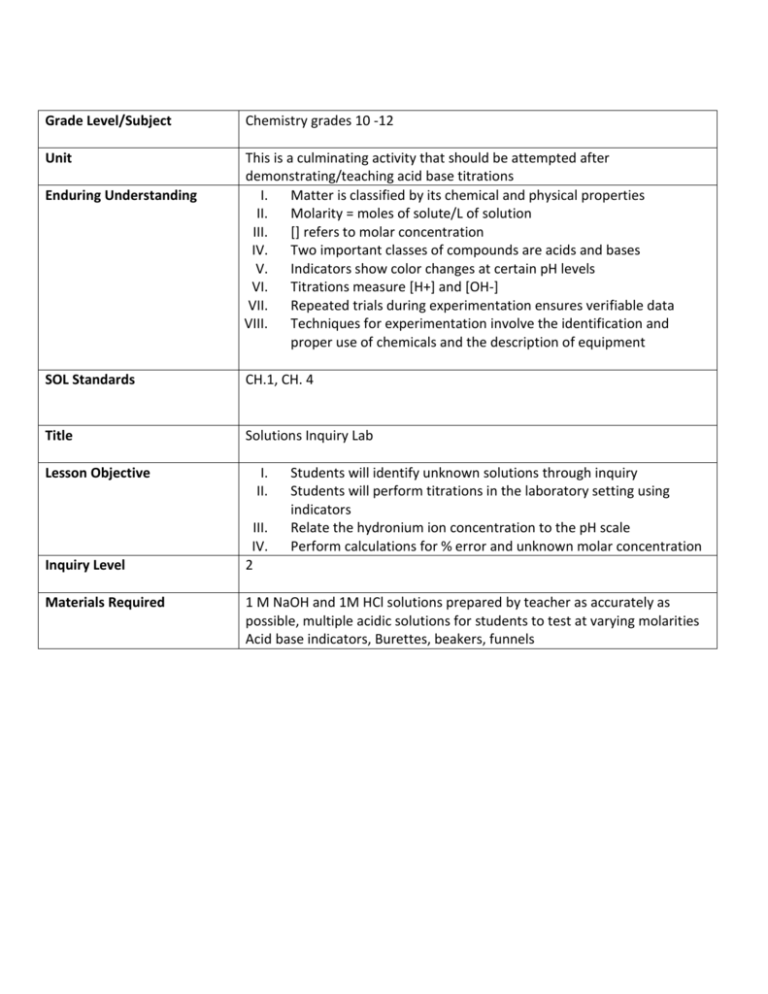
Grade Level/Subject Chemistry grades 10 -12 Unit This is a culminating activity that should be attempted after demonstrating/teaching acid base titrations I. Matter is classified by its chemical and physical properties II. Molarity = moles of solute/L of solution III. [] refers to molar concentration IV. Two important classes of compounds are acids and bases V. Indicators show color changes at certain pH levels VI. Titrations measure [H+] and [OH-] VII. Repeated trials during experimentation ensures verifiable data VIII. Techniques for experimentation involve the identification and proper use of chemicals and the description of equipment Enduring Understanding SOL Standards CH.1, CH. 4 Title Solutions Inquiry Lab Lesson Objective Inquiry Level Materials Required I. II. III. IV. 2 Students will identify unknown solutions through inquiry Students will perform titrations in the laboratory setting using indicators Relate the hydronium ion concentration to the pH scale Perform calculations for % error and unknown molar concentration 1 M NaOH and 1M HCl solutions prepared by teacher as accurately as possible, multiple acidic solutions for students to test at varying molarities Acid base indicators, Burettes, beakers, funnels Acid Base Titration Inquiry Lab Refer to your notes and the Titration of Vinegar with Sodium Hydroxide lab before beginning this investigation. An acid-base titration is a neutralization reaction that is performed in the lab in order to determine an unknown concentration of acid or base. The moles of acid will equal the moles of base at the equivalence point. Here's how to perform the calculation to find your unknown. For example, if you are titrating hydrochloric acid with sodium hydroxide: HCl + NaOH → NaCl + H2O You can see from the equation there is a 1:1 molar ratio between HCl and NaOH. If you know that titrating 50.00 ml of an HCl solution requires 25.00 ml of 1.00 M NaOH, you can calculate the concentration of hydrochloric acid, [HCl]. Based on the molar ratio between HCl and NaOH you know that at the equivalence point: moles HCl = moles NaOH MHCl x volumeHCl = MNaOH x volumeNaOH MHCl = MNaOH x volumeNaOH / volumeHCl MHCl = 25.00 ml x 1.00 M / 50.00 ml MHCl = 0.50 M HCl For this investigation you will be given three solutions of “unknown” pH. You and your partner(s) are to plan and preform a titration using 1M NaOH and/or 1 M HCl(provided by teacher) to determine the pH of the three unknown solutions. Sketch and label a titration apparatus in you lab books. Create a procedure and record in your lab book, and preform the investigation; record calculations to determine pH of each sample in your lab book. Finally, check on blackboard tonight to discover the actual pH of the unknown solutions, and answer the following questions for homework. Remember you are working with acids and bases. Follow all safety protoco ls. Questions: I. II. How would you go about checking the accuracy of your data? Calculate your % error and explain possible sources of error. Error is a measure of the accuracy of the values in your experiment. It is important to be able to calculate experimental error, but there is more than one way to calculate and express it. Here are the most common ways to calculate experimental error: Error Relative Error Percent Error Error = Experimental Value - Known Value Relative Error = Error / Known Value % Error = Relative Error x 100% Let's say a researcher measures the mass of a sample to be 5.51 g. The actual mass of the sample is known to be 5.80 g. Calculate the error of the measurement. Experimental Value = 5.51 g Known Value = 5.80 g Error = Experimental Value - Known Value Error = 5.51 g - 5.80 g = - 0.29 g Relative Error = Error / Known Value Relative Error = - 0.29 g / 5.80 g = - 0.050 % Error = Relative Error x 100% % Error = - 0.050 x 100% = - 5.0% Although oxygen gas is colorless, the liquid and solid forms of oxygen are blue











