Event location - LAU | Student Life
advertisement

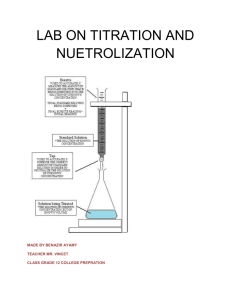
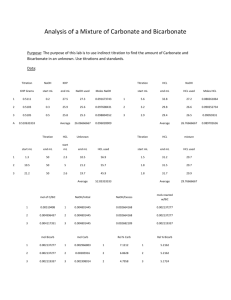
2011 Was declared by UNESCO and IUPAC as the International Year of Chemistry world-wide. LAU celebrates the International Year of Chemistry by hosting the first Acid – Base Titrations Competition Event location: Science Building of Lebanese American University Byblos Event date: Saturday, April 2nd, 2011 Participants: up to 20 (age group 2nd, 1ere, high) Evaluation criteria: Accuracy, Precision, Technique Registration deadline: November 30, 2010 Participants need to bring their own laboratory coat and safety goggles to the event. The participants can prepare for the event. A typical titration procedure is outlined below. On the day of the competition, the participants will perform the titrations three times in the laboratories of LAU. The winners of the competition will be announced at the Annual Science and Arts Fair on May 5/6, 2011. 1 Procedure: We will use the titration equation relating CaVa to CbVb which allows us to determine what combination of acid and base results in neutralization. Calculation example: What volume of 1 M Al(OH)3 would be required to neutralize 50. mL of 2.0 M H2SO4? Solution: CA = 2.0 mol/L, VA = 0.050 L, #H+ = 2; CB = 1.0 mol/L, #OH = 3, VB = ? (2.0 mol/L)(2)(0.050 L) = (1 mol/L)(3)(VB), thus VB = 0.067 L or 67 mL Titration assembly: Procedure of a typical acid base titration 1. Gather together a burette, a squeeze bottle containing distilled water, a 100 mL beaker, a 50 mL beaker. Rinse all equipment well with distilled water. 2. Place 0.2 M HCl in the 50 mL beaker. Rinse the burette with acid (don’t forget to rinse the tip). Fill the burette to the 0 mL mark with HCl. (Ensure that there are no air bubbles in the tip of the burette). 3. Using a 10.00 ml pipette, transfer 10.00 mL of an NaOH solution( that will be given to you) into the 250 mL Erlenmeyer flask. 4. Add 5 drops of phenolphthalein to the NaOH. (the solution will turn pink) 5. Rinse the inside walls of the Erlenmeyer with distilled water. 2 6. Now start your titration by adding HCl dropwise (from the burette) over the NaOH solution in the Erlenmeyer flask. You have to stir continuously the solution. If no magnetic stirrer is available you may shake the flask continuously with one hand, while doing the titrations with the other hand. 7. Keep on adding HCl till the pink color disappears ;this indicates the complete neutralization. (make sure not to overshoot the titration end point) 8. Record the volume of HCl delivered from the burette. 9. Repeat this procedure 2 more times in order to get more accurate results. 9. Take the average value of the volume of HCl in order to calculate the concentration of NaOH which was given to you. (value should be given to 4 decimal places). 10. Do clear calculation and submit your results . 11. Rinse all the glassware well with tap water. Table of result: Number written on your flask is: Volume of HCl Trial # 1 2 3 VHCl Average Volume Concentration of NaOH 3