Report Sheet
advertisement

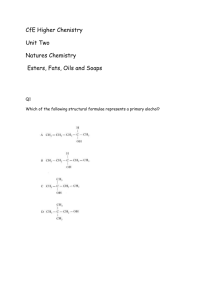
Report Sheet Experiment 4 Part 1 : Aldehydes and Ketones I. Data and Observations Unknown number _______ A1. 2,4-Dinitrophenylhydrazones Observations for benzaldehyde: Observations for methyl ethyl ketone: Observations for unknown: A2. Semicarbazones Observations for benzaldehyde: Observations for methyl ethyl ketone: Observations for unknown: Clayton State University CHEM 2412L Dr. Susan F. Hornbuckle Name __________________ Clayton State University CHEM 2412L Dr. Susan F. Hornbuckle B1. Chromic acid regent Observations for propanaldehyde: Observations for benzaldehyde: Observations for methyl ethyl ketone: Observations for unknown: C. Iodoform Test Observations for methyl ethyl ketone: Observations for unknown: II. Data Analysis 1. Attach a copy of your unknown compound FTIR spectrum to this report. Identify as many peaks as possible in the spectrum. 2. Identify the functional group present in your unknown. Explain how you came to this conclusion using your observations and spectroscopic data. III. Questions 1. Give an example of a simple chemical test that would distinguish pentanal from 2-pentanone. Explain. Clayton State University CHEM 2412L Dr. Susan F. Hornbuckle 2. How would you distinguish between 2-pentanone and 2-pentanol by use of infrared spectroscopy only? Explain. Experiment 4 Part 2: Esterification Report Sheet I. Data and Observations Tube # Carboxylic Acid Alcohol 1 Formic Acid (methanoic acid) Isobutyl Alcohol 2 Acetic Acid (ethanoic acid) Amyl (1-pentanol) 3 Acetic Acid (ethanoic acid) 1-octanol 4 Acetic Acid (ethanoic acid) 1-propanol 5 Propanoic Acid Isobutyl Alcohol 6 Cinnamic Acid Methanol 7 Cinnamic Acid Ethanol 8 Salicylic Acid Methanol 9 ____ - 1 ____ - 2 10 ____ - 1 ____ - 2 Scent Clayton State University CHEM 2412L Dr. Susan F. Hornbuckle Note any observations in color of each ester. Tube # Observations 1 2 3 4 5 6 7 8 9 10 Note which ester you think your unknowns are based strictly on physical appearance and scent Tube # Carboxylic Acid Alcohol 9 ____ - 1 ____ - 2 10 ____ - 1 ____ - 2 Name of Ester II. Reactions (Draw the reactions taking place in each of the 8 known reactions in this experiment. You do not need to show a mechanism. Also provide the name of the ester produced for each of the 8 known reactions) Clayton State University CHEM 2412L Dr. Susan F. Hornbuckle III. Data Analysis 1. For the reaction in test tube #1 calculate the limiting reagent and the theoretical yield. Assume that there are 20 drops in 1 mL. Show your work for full credit. 2. Match the experiments in test tubes 1, 4, and 5 with the 1H-NMR spectra A, B, and C below. For each 1H-NMR spectrum label which sets of hydrogens in the structure corresponds to which signal in the spectrum. Clayton State University CHEM 2412L Dr. Susan F. Hornbuckle IV. Questions 3. Esterification is a highly reversible reaction. Which fundamental principle in chemistry should be utilized to improve the yield of the ester? Give two ways that this can be accomplished. 4. We know that the esterification reaction is in equilibrium. Does water participate in the equilibrium constant? Back up your answer with an explanation. 5. What is the purpose of using a saturated sodium chloride solution in this experiment?