WS Bonding Comparison
advertisement
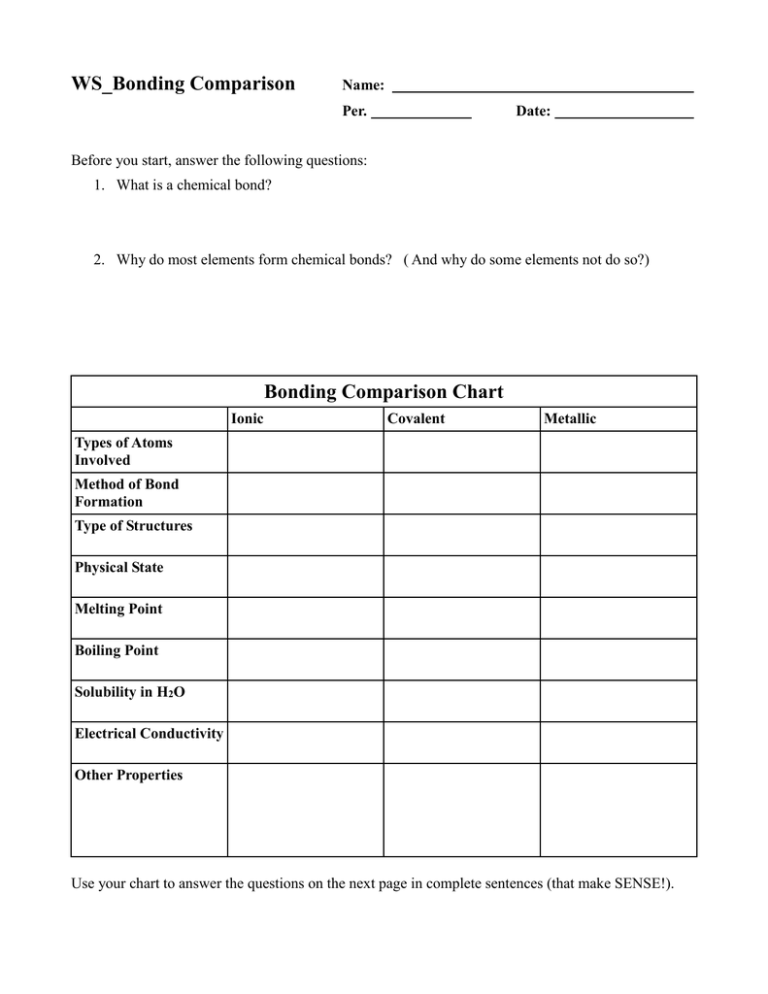
WS_Bonding Comparison Name: Per. Date: Before you start, answer the following questions: 1. What is a chemical bond? 2. Why do most elements form chemical bonds? ( And why do some elements not do so?) Bonding Comparison Chart Ionic Covalent Metallic Types of Atoms Involved Method of Bond Formation Type of Structures Physical State Melting Point Boiling Point Solubility in H2O Electrical Conductivity Other Properties Use your chart to answer the questions on the next page in complete sentences (that make SENSE!). 1. What types of atoms typically form ionic bonds? 2. What types of atoms typically form covalent bonds? 3. How are ionic bonds formed and what type of structure is generally formed? 4. What are the typical properties of ionic substances? (Include at least five properties) 5. Draw a Bohr representation of ionic bonding showing before and after for atoms as well as ions. 6. Describe the Electron Sea concept, use the term “valence electron.” 7. Draw a representation of metallic bonding. 8. What are two fundamental differences between covalent bonding and ionic bonding and the structures they produce?