bond aromatic
advertisement
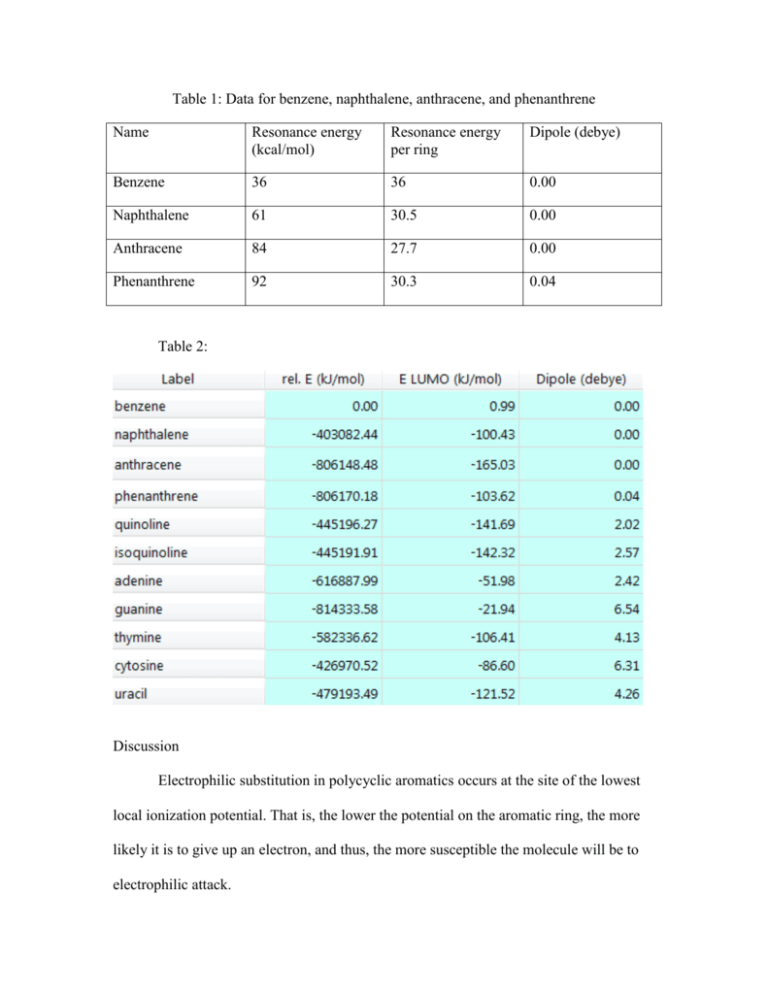
Table 1: Data for benzene, naphthalene, anthracene, and phenanthrene Name Resonance energy (kcal/mol) Resonance energy per ring Dipole (debye) Benzene 36 36 0.00 Naphthalene 61 30.5 0.00 Anthracene 84 27.7 0.00 Phenanthrene 92 30.3 0.04 Table 2: Discussion Electrophilic substitution in polycyclic aromatics occurs at the site of the lowest local ionization potential. That is, the lower the potential on the aromatic ring, the more likely it is to give up an electron, and thus, the more susceptible the molecule will be to electrophilic attack. While benzene, naphthalene, anthracene, and phenanthrene molecules exhibit similarities—in that these are all polycyclic aromatic compounds that have a number of benzene rings fused together, and thus, have aromatic characters—some molecules have more localization of electrons (i.e., a denser electron area) than others. This can be confirmed by the dipole value and resonance energy of each molecule as shown in Table 1. According to the polarity and resonance energy, phenanthrene is the only molecule that has polarity character with a dipole value of 0.004 debye. This suggests that phenanthrene must have both electron rich areas and electron poor (deficient) areas. In an electron deficient area, the electron will be given up more easily than in an electron rich area; therefore, phenanthrene is the most susceptible to an electrophilic attack among the four molecules. Among the remaining three molecules, anthracene will be the next most susceptible molecule, followed by naphthalene and benzene. Comparing resonance energy, the anthracene molecule has the lowest stabilization energy at 27.7kcal/mol followed by naphthalene with 30.5kcal/mol and benzene with 36kcal/mol. This suggests that anthracene is the least stable molecule among the three. The varying resonance energies among these molecules are due to different bond lengths; the electrons for C–C bonding are distributed equally between each of the six carbon atoms for benzene, which has equal bond lengths. Other molecules, however, do not share electrons equally, which makes the bond unsymmetrical, and therefore, there will be an area where electrons are more localized and deficient. Figure 2 shows that electrons are concentrated to small areas both on quinoline and isoquinoline. This makes other areas of these compounds relatively electron deficient. Among the two molecules, the nitrogen atom in isoquinoline is relatively more distant from the adjacent benzene ring than the nitrogen atom in quinoline. The most electron deficient site of the isoquinoline is one carbon farther from the nitrogen atom, which makes isoquinoline more electron deficient than quinoline. Therefore, the electrophilic substitution on isoquinoline will occur faster than on quinoline. Thus, the order of the rate of relative electrophilic substitution will be isoquinoline, followed by quinoline, and finally napthalene (where there is relatively no electron localization). This result is shown in Table 2. Isoquinoline has the highest dipole moment, suggesting that it has the most polarity or electron localization; therefore, isoquinoline is the most susceptible to electrophilic attack with 2.57 debye, followed by quinoline with 2.02 debye, and naphthalene with 0.00 debye. The inductive effect applies when there are substituents in which nitrogen, oxygen, and halogen atoms form sigma-bonds to the aromatic ring, and thus, exert an inductive electron withdrawal, which deactivates the ring. As seen in Figure 3, local ionization potential maps of adenine, guanine, thymine, cytosine, uracil, and guanine have the most electron deficient sites (indicated by the blue area). These sites are withdrawn by electronegative atoms of oxygen and nitrogen, followed by cytosine, uracil, thymine, and adenine. This is once again confirmed by the dipole moment shown in Table 2. Guanine has the highest dipole moment of 6.54 debye, illustrating that it has the strongest polarity and will deactivate the molecule by withdrawing electron density. Cytosine follows with the value of 6.31 debye, uracil with 4.26 debye, thymine with 4.13 debye, and adenine with 2.42 debye. This can be attributed to different resonance stabilization energy. As shown in the ionization potential maps in Figure 1, there is no distinctive difference among benzene, naphthalene, anthracene, and phenanthrene since the electrons are well spread out along the conjugated pi system. END OF SAMPLE Copyright notice: All information contained in this document is copyrighted. No part of this document or any of its contents may be reproduced, copied, modified or adapted, without the prior written consent of the author.