Heterocyclic Chemistry
advertisement

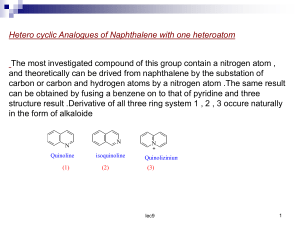
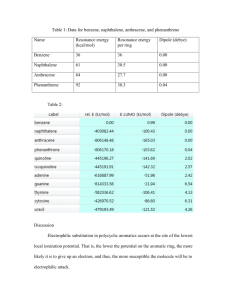
Heterocyclic Chemistry N S O Benzopyridines Quinoline and Isoquinoline: Heterocyclic Chemistry Introduction: N N Quinoline Benzo[b]pyridine Isoquinoline Benzo[c]pyridine N Quinolizinium ion Benzo[a]pyridinium Heterocyclic Chemistry Introduction : Quninoline and isoquinoline are two fused heterocycles derived by fusion of pyridine ring with a benzene ring. Quinoline is high boiling liquid (b.p. 237°C) and smells like pyridine while isoquinoline is a low melting solid (m.p. 26.5°C, b.p. 243°C). Both quinoline and isoquinoline are planar 10πelectron aromatic systems in which all atoms are sp2 hybridized and contribute one electron each in orthogonal p-orbitals for delocalization over the rings with resonance energies of 198 and 143 KJ/mol respectively Heterocyclic Chemistry Resonance structures of Quinoline and isoquinoline Occurrence of quinoline Both ring systems occur naturally and were originally isolated from coal tar. Heterocyclic Chemistry Occurrence isoquinoline Emetine is a drug used as both an anti-protozoal and to induce vomiting It is a toxic crystalline alkaloid It inhibits protein synthesis and may inhibit ascorbic acid biosynthesis it serves some interest in the study of certain yeasts Heterocyclic Chemistry Basicity Quinoline and isoquinoline are weak bases but slightly more basic than pyridine (why?) but less basic than anilines since the nitrogen in quinoline and isoquinoline is more electronegative being sp2 hybridized compared to sp3 hybridized nitrogen of anilines. Heterocyclic Chemistry Preparation of Quinoline 1. Friedländer Synthesis O O CH + H2O, NaOH, 50 ºC H N NH2 2-Aminobenzenecarboxaldehyde Enolizable carbonyl compound 85% Paul Friedländer (1857 - 1923) Preparation of Quinoline 2- Skraup’s Synthesis General Equation: H CH2 CH 2OH CHOH CH 2OH Glycerol O CH 2 NH2 CH -H2O CH 2 C H N H O Acroline -H2O C6H5NO2 Oxidation N N H Preparation of Isoquinoline Bischler-Napieralski Synthesis POCl3, P2O5, 100 ºC NH R O H + N -H2O Pd, 200 ºC, - H2 R N R Electrophilic aromatic substitution Isoquinoline in quinoline and The nitrogen of the quinoline and isoquinoline has deactivating effect on the ring towards electrophilic substitution as in case of pyridine. However electrophilic substitution of quinoline and isoquinoline requires less vigorous conditions than pyridine. Consequently electrophilic aromatic substitution occurs at the benzene ring at positions 5 and 8. Heterocyclic Chemistry Electrophilic aromatic substitution in quinoline and isoquinoline Electrophilic aromatic substitution Isoquinoline in quinoline However, nitration can take place at pyridine ring using nitric acid and acetic anhydride at position 3 in case of quinoline and at position 4 in case of isoquinoline and Nucleophilic aromatic substitution in quinolines and Isoquinolines Quinoline and isoquinoline undergo facile nucleophilic substitution as in pyridine. Quinoline undergoes Chichibabin reaction to give 2-aminoquinoline while isoquinoline undergoes Chichibabin reaction to give 1-amino isoquinoline . Isoquinoline undergoes substitution faster than quinoline. The reaction proceeds in a manner analogues to pyridine. Heterocyclic Chemistry Reduction of quinoline The pyridine ring is more easily reduced Quinoline can be selectively reduced at 1,2-bond by reaction with lithium aluminium hydride but the 1,2-dihydro quinolines are unstable and disproportionate easily to give quinoline and 1,2,3,4-tetrahydroquinoline. Quinoline can be converted to 1,2,3,4-tetrahydroquinoline by catalytic hydrogenation or with tin and hydrochloric acid Heterocyclic Chemistry Reduction of isoquinoline The pyridine ring is more easily reduced Isoquinoline can also be converted to 1,2-dihydro or 1,2,3,4-tetrahydroisoquinoline with diethyl aluminium hydride and sodium-ethanol, respectively Oxidations Quinoline and isoquinoline undergo oxidative cleavage with alkalian potassium permangnate to give pyridine-2,3dicarboxylic acid and pyridine-3,4-dicarboxylic acid respectively. However, pyridine-2,3-dicarboxylic acid is not stable and undergoes decarboxylation to give nicotinic acid. Quinoline and isoquinoline both form N-oxides when treated with hydrogen peroxide in acetic acid or with organic peracids. Thanks for your attention Heterocyclic Chemistry