Week 5 - Structures and Bonding Test
Year 10 iGCSE Structures and Bonding Test
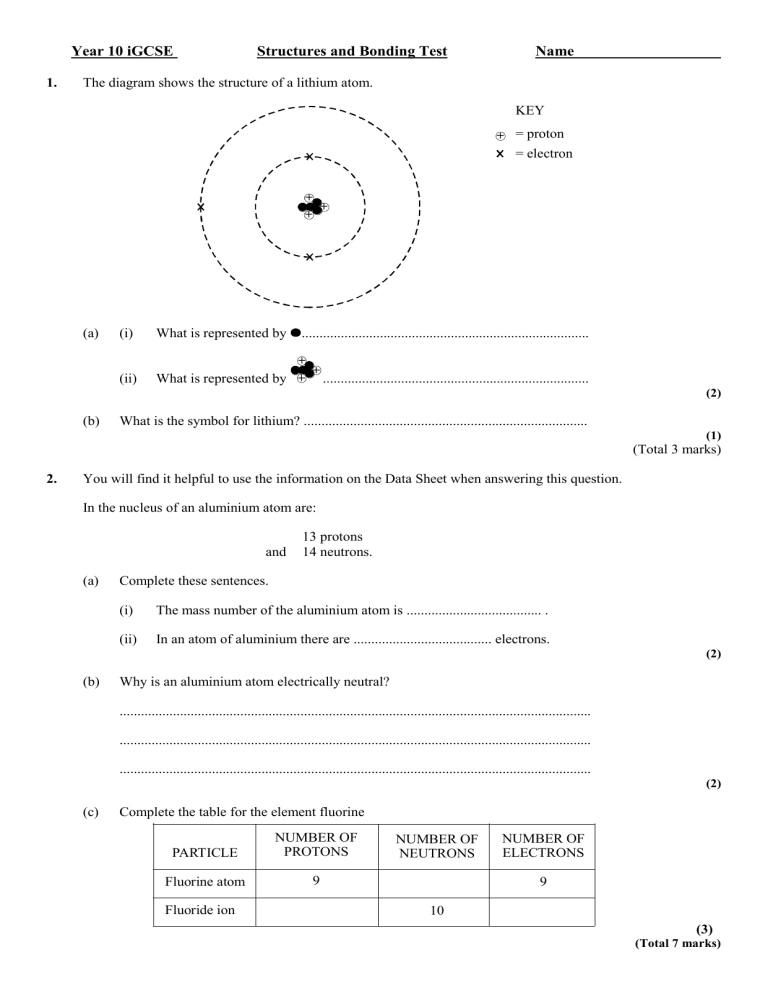
1. The diagram shows the structure of a lithium atom.
Name
KEY
+
= proton
= electron
+
+
+
(a) (i) What is represented by .................................................................................
(ii) What is represented by
+
+
+
...........................................................................
(2)
(b) What is the symbol for lithium? ................................................................................
2.
You will find it helpful to use the information on the Data Sheet when answering this question.
In the nucleus of an aluminium atom are:
13 protons and 14 neutrons.
(a) Complete these sentences.
(i) The mass number of the aluminium atom is ...................................... .
(ii) In an atom of aluminium there are ....................................... electrons.
(1)
(Total 3 marks)
(2)
(b) Why is an aluminium atom electrically neutral?
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
(2)
(c) Complete the table for the element fluorine
PARTICLE
NUMBER OF
PROTONS
Fluorine atom 9
Fluoride ion
NUMBER OF
NEUTRONS
10
NUMBER OF
ELECTRONS
9
(3)
(Total 7 marks)
3.
The diagram shows one molecule of the compound ammonia.
H H
N
H
Write down everything that the diagram tells you about each molecule of ammonia.
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
4. Magnesium oxide is a compound, made up of magnesium ions and oxide ions.
Mg O Mg
O Mg O
Mg O Mg
(a) What is the charge on each magnesium ion? .............................................................
(b) Explain how the magnesium ions get this charge.
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
(Total 4 marks)
(1)
5. Chlorine will combine with the non-metal element, carbon, to form this molecular compound.
Cl
Cl C Cl
Cl
(a) What is the type of bond in this molecule?
.....................................................................................................................................
(2)
(Total 3 marks)
(b) Explain how these bonds are formed. (You may use a diagram).
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
(2)
(Total 3 marks)
6.
(a) (i) Complete the drawing to show the electron structure of a hydrogen fluoride molecule. Draw electrons as dots or crosses.
(ii) Explain why hydrogen fluoride is a gas at room temperature.
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
(1)
(2)
(Total 3 marks)
7.
Complete the following table about calcium oxide and carbon dioxide.
Calcium oxide
CaO Formula
Appearance at room temperature
Solubility
Type of bonds
One use slightly soluble in water to neutralise acidic soils
[2]
[1]
Carbon dioxide colourless gas covalent
[1]
[1]
[1]
(Total 6 marks)
8. Graphite is a non-metal.
Use the information to explain why graphite conducts electricity.
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
.....................................................................................................................................
(3)
Total 32 marks











