Chapter 4.1 Atomic Theory and Bonding
advertisement

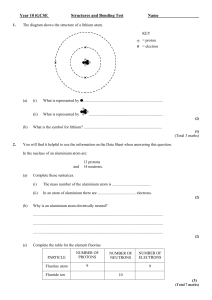
Name: Period: Science 10 Pre – Test for Chemistry /86 Answer all the questions in preparation for your unit test, remember, all the material covered in the pre – test will be on the unit test. This is an open book assignment. Chapter 4.1 Atomic Theory and Bonding 1. Fill in the table regarding sub atomic particles (6 marks) Sub atomic particle Charge Proton Electron Neutron 2. What two things does the atomic number tell you? (2 marks) 3. What is the number of neutrons in an atom of Sulphur? (1 mark) 4. How does an atom become an ion? (1 mark) 5. Why do non-metals become negative ions? (1 mark) 6. How many valence electrons are in the atom below? (1 mark) Location in the atom 7. What is the relationship between valence electrons and families? (1mark) 8. Draw a Bohr diagram of a fluorine atom and ion (2 marks) Fluorine Atom Fluorine Ion 9. Draw a Lewis diagram of a fluorine atom and ion (2 marks) Fluorine Atom Fluorine Ion 10. What types of atoms for ionic bonds? (2 marks) 11. What types of atoms form covalent bonds? (2 marks) 12. What are the seven diatomic molecules? (7 marks) 13. How many lone pairs and bonding pairs are in this compound? (2 marks) Lone pairsBonding Pairs- 4.2 Names and Formulas of Compounds 14. Write the name and formulas for the following compounds. (14 marks) Chemical name Formula # of atoms Sodium hydroxide Fe(OH)2 Nickel II hydrogen carbonate Cu(HSO3)2 Ammonium chromate Sr3(PO4)2 Copper II nitride 15. Write the formula or name for the following covalent compounds. (4marks) Formula Name Sulfur trioxide Dinitrogen monoxide PBr3 N2S3 4.3 Chemical Equations 16. Balance the following chemical equations. (4 marks) 17. Write the skeletal equations for the following chemical reactions and balance them. Be sure to check your formulas carefully before you begin to balance. (3 marks) Chapter 5.1 Acids and Bases 18. What is the color of each of these acid-base indicators in the following solutions? (2marks) a. Methyl orange in stomach acid ________________ b. Bromothymol blue in banana juice _____________ 19. Name the following acids. (3 marks) a. HBr _____________________________ b. H2SO4 ___________________________ c. HClO4 ___________________________ 20. Identify the following as an acid, base or salt.(3 marks) 5.2 Salts 21. Identify each of the following as an acid, a base, a salt, a metal oxide, or a non-metal oxide. (5 marks) 22. Complete the following statements. (4 marks) a. A metal oxide combines with water to form a (n) ______________________. b. A non-metal oxide combines with water to form a (n) ________________________. c. An acid and base combine to form a (n) ______________________ and _______________________. 5.3 Organic Compounds 23. Identify which of the following are organic compounds. (4 marks) Compound Organic or Inorganic CaCO3 CaH2 CH4 H2CO3 6.1 Types of Chemical Reactions 24. Identify each reaction as synthesis, decomposition, single replacement, double replacement, neutralization, or combustion. (6 marks) 6.2 Factors Affecting the Rate of Chemical Reactions 25. Explain how the following increase the rate of a chemical reaction. (4 marks) Factor Temperature Surface area Concentration Catalyst Explanation