Chemistry
advertisement

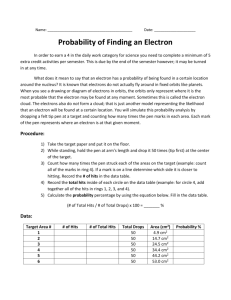
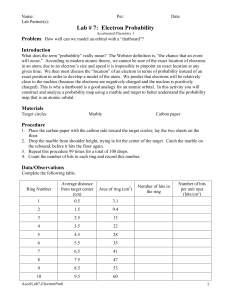
Chemistry Laboratory Investigation # _____ Name _______________________ Period _________ Date _________ Electron Probabilities INTRODUCTION Could you determine the exact position and momentum of a baseball as it soared through the air? Of course you could – by taking a timed series of snapshots of the baseball as it moved. Why then can’t scientists follow a similar procedure to determine the position and momentum of an electron? You can see a moving baseball or its image because of the light bouncing off the baseball. The effect of the light on the position or the momentum of the baseball is negligible. By contrast, an electron has such an extremely small mass that light disturbs it in an unpredictable way. How can the position and momentum of an electron be determined? Knowledge of the behavior of electrons in the atom comes from the theoretical work done in the 1920s by the German physicist Werner Heisenberg (1901-1976) and the Austrian physicist Erwin Schrödinger (1887-1961). Heisenberg postulated that it was impossible to determine exactly both the position and the momentum of an electron at the same instant. Heisenberg deduced that the more certain you know the position of the electron, the less certain you are about its momentum, and vice versa. Because the exact position and momentum can never be established at any given time, the exact path of an electron through the electron cloud can never be determined. Instead the modern model of the atom, called the quantum-mechanical model, gives the probabilities of finding an electron in a particular region around the nucleus. In this investigation, you will model the probable locations of electrons around the nucleus of an atom. You will use a marble and a target to represent electrons to help you visualize regions of both high and low electron density. PROCEDURE 1. Measure the distance from the nucleus to the edge of each ring. Record your values in your data table. 2. Place the target on the floor. Drop a marker from about chin height onto the target. Aim for the center of the target. 3. Repeat this for a total of 54 drops. 4. Count the number of hits in each ring. Record your values in your data table. Data & Analysis Ring # Distance from nucleus to edge of ring (cm) Number of hits in each ring 1 2 3 4 5 Calculate the area of each full circle. A = r2. Ring # Area of full circle (cm2) 1 2 3 4 5 Calculate the area of each ring. For example, the area of just ring 2 is the area of ring 2 minus ring 1, etc. Ring # Area of each ring (cm2) 1 2 3 4 5 Calculate the density of hits within each ring. D = # hits/area of ring. Ring # Density of Hits (hits/cm2) 1 2 3 4 5 GRAPH Prepare a graph of your density of hits vs.distance from the nucleus. Use graph paper Use a ruler Label your x-axis “Distance from nucleus (cm)”. Choose an appropriate scale. Label your y-axis “Density of Hits (hits/cm2)”. Choose an appropriate scale. Plot your points. Connect your points with a smooth line/curve. Give your graph a descriptive title. Staple your graph to the back of this lab. Extensions (Please write in complete sentences.) 1. Which element is being represented in this activity? How do you know? 2. Which orbital shape do the results of this experiment best represent? To which quantum number does this correspond? 3. Where is the density of hits the highest? 4. Explain how your answer to #3 supports the Aufbau Principle. 5. If you had used 108 drops instead of 54, what changes (if any) would you predict for your results? 6. Even though the Bohr model is outdated and incorrect, why do you think most people persist in visualizing the atom this way as opposed to accepting the quantum–mechanical model? Target