Study Guide key
advertisement

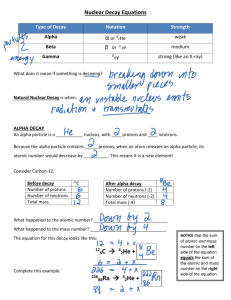
H Study Guide Ch.4 & 25 1. 234 90 Th 234 91 2. 210 83 Bi 0 −1𝑒 3. 234 91 Pa* 4. 10 5 B + 01 n 5. 14 6 C 6. 234 91 7. 23 12 8. 210 82𝑃𝑏 14 7 234 91 → + 7 3𝐿𝑖 gama decay 4 2 He + + 4 2 4 2 neutron bombardment resulting in alpha decay beta decay 0 −1𝑒 19 10𝑁𝑒 + beta decay Pa + 00𝛾 230 89𝐴𝑐 0 −1𝑒 beta decay 0 −1𝑒 + 210 84𝑃𝑜 N + Pa Mg Pa + He He + 00 alpha decay alpha decay w/gamma emission 210 83𝐵𝑖 9. The neutron to proton ratio that correlates with the band of stability. Radioactivity results when unstable nuclei emit energy in order to gain stability. 10. Fusion gives off more energy. Nuclear reactions give off more energy. Ch 4 36. Charge= -1; mass= 9.11 x10-28g 37. Strengths: Rutherford’s model explained the results of the gold-foil experiment and why an atom is electrically neutral. Weakness: the model could not account for the total mass of an atom or the arrangement of the electrons. 38. Protons and neutrons; net positive charge equal to the number of protons 39. attraction to the positively charged nucleus 41. the plum pudding model 42. the α particles were deflected by the positively charged gold nuclei 43. protons and neutrons 44. they are all equal 46. the number of positively charged protons equals the number of negatively charged electrons 48. differ: number of neutrons, masses; similar: chemical properties, number of protons and electrons 50. the superscript represents the mass number (40) and the subscript represents the atomic number (19) 51. number of n0 = mass number – atomic number 56. Particle α β γ Symbol 4 He 2 0 β −1 0 γ 0 Mass (amu) 4 Charge +2 1/1840 -1 0 0 57. α, mass number decreases by 4; β, no change in mass number; γ, no change in mass number 62. mass number is 200 64a. 132 Cs, e- =55, p+ =55, n0 =77 55 65a. Ga-64, e- =31, p+ =31, n0 =33 68. 51.99 amu (52.0 amu with sf) 82. 28 Si 14 29 Si 14 30 Si 14 Ch.25 35. 1. Atoms; 2. Stable; 3. Unstable; 4. Do not decay; 5. -9. Alpha decay, beta decay, gamma emission, electron capture, positron emission 37. nuclear reactions release more energy per mole. Nuclear reactions incolve neutrons and protons, whereas chemical reactions involve electrons 38. a: 2, 4, 5; b: 1; c: 3,6 42. the strong nuclear force binds nucleons together 48. transmutation is the changing of an atoms nucleus such that a new element is formed. Most, but not all nuclear reactions are transmutations 50. radioactive, short half-lives, alpha emitters 54. difference between mass of the nucleon’s summed separately and the actual mass of the nucleus 57. a chain reaction occurs when a reaction produces one or more of the particles needed as a reactant. An example is the fission of U-235 by absorption of a neutron 79. 9.2 half-lives; 0.625 mg