Chemistry I: 3-4 Practice and Review Name________________________________ Period_______Date_____________________
advertisement

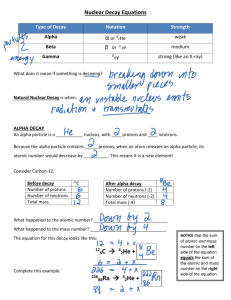
Chemistry I: 3-4 Practice and Review Name________________________________ Changes in the Nucleus Period_______Date_____________________ 1. Nuclear reactions change the composition of an atom’s _____________________. 2. The attractive force that overcomes the electric repulsion between protons is the ______________ _________________ force. 3. Almost all the atoms you encounter have _________________ nuclei. 4. All nuclei with atomic numbers greater than 83 are __________________________. 5. Alpha, beta and gamma radiation are distinguished by their charge, _________________, and penetrating power. 6. When an atom emits alpha, beta or gamma radiation, it is undergoing _________________ decay. Answer each of the following in the space provided: 7. Why is carbon dating not useful for artifacts made entirely of metal? _______________________________________________________________________________ _______________________________________________________________________________ 8. Compare the penetrating power of alpha, beta and gamma radiation: _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ 9. Why do nuclei need neutrons to be stable? ____________________________________________ _______________________________________________________________________________ 10. List 2 types of nuclear reactions other than radioactive decay: ____________________________ 11. In any radioactive decay, the sum of the mass numbers and atomic numbers must be ___________ before and after the reaction. a) greater b) the same c) less d) unpredictable 12. The most dangerous form of radiation to the human body is a) beta radiation b) gamma radiation c) alpha radiation d) They are equally dangerous 13. To be stable, atoms with more than 20 protons need increasingly more a) neutrons than protons b) electrons than protons c) electrons than neutrons d) protons than neutrons 14. The sun produces energy by means of a) gamma radiation b) beta decay c) alpha decay d) nuclear fusion 15. 226 88 Ra 16. 14 6C 27 Al 17. __ 222 86 Rn 14 7N + 4 __ He + ______ + ______ 12 C 6 + ______ Write a nuclear equation for the following reactions: 18. alpha decay of polonium-210 _______________________________________________________ 19. beta decay of tritium ( 31H ) _________________________________________________________ 20. alpha decay of thorium-230 ________________________________________________________ 21. alpha decay of 231 91 Pa _____________________________________________________________ 22. What particle bombards Beryllium-9 if it forms carbon-12 and neutrons? Write the equation: ______________________________________________________________________________ 23. beta decay of 165 61 Pm _____________________________________________________________ 24. What isotope is bombarded with alpha particles to produce oxygen-17 and hydrogen-1? Write the equation: ______________________________________________________________________________ 25. alpha decay of 146 62 Sm ____________________________________________________________ 26. beta decay of 198 85 At _____________________________________________________________ 27. Write the equation of the bombardment of boron-10 with neutrons to produce Li-7 and another particle. ______________________________________________________________________________ 28. beta decay of 152 54 Xe _____________________________________________________________