HomeworkCh_13Answers
advertisement

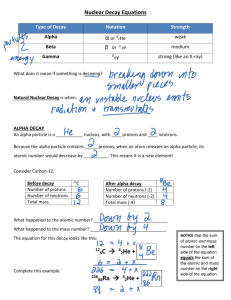
Chapter 13 1. How many protons and neutrons are in each of the following nuclides? a. Po-209, protons:84 b. 23290Th, protons:90 c. Bi-209, protons:83 d. 20782Pb, protons:82 neutrons:125 neutrons:142 neutrons:126 neutrons:125 2. Write each of the following nuclides in the subscripted-superscripted form. For example, H-2 would be written as 21H. a. b. c. d. C-14, 146C Cs-133, 13355Cs Li-7, 73Li Bi-209, 20983Bi 3. What does it mean to be radioactive? Radioactive is a nucleus that is inherently unstable and releases energy and/or particles. 4. Which of the following statements are True for chemical reactions? a. The number and types of atoms are the same in the products and the reactants. True b. Different isotopes of the same element behave similarly. True c. Only the valence electrons are involved. True d. New elements are often formed. False 5. Which of the statements in Question 4 are True for nuclear reactions? a. b. c. d. False True True True 6. Fill in the missing product in the following nuclear reactions. a. 20881Tl 20882Pb + 0-1beta, the atomic number for the product decreased by one so the reaction must have emitted a beta particle b. 263106Sg 42He + 259104Rf, the atomic number decreased by four and the atomic weight by 2 c. 21484Po 0-1beta + 21483Bi d. 8738Sr 00gamma + 8738Sr, no change atomic number or atomic weight 7. A portion of the radioactive decay series that starts with Th-232 is as follows. For each step, write the product. Th-232 (23290Th) (goes through alpha decay) 22888Ra + 42He (goes through beta decay) 22887Fr + 0-1beta (goes through beta decay) 22886Rn + 0-1beta (goes through alpha decay) 22484Po + 42He (goes through alpha decay) 22082Pb + 42He (goes through alpha decay) 21680Hg + 42He 8. Two common units for measuring radioactivity are the Becquerel and the Curie. Why are these names appropriate? Both Becquerel and Curie were scientists who pioneered the study of nuclear reactions and radioactivity. 9. Why do nuclear reactions release so much more energy than chemical reactions? Matter can be converted into energy during nuclear reactions. A very small amount of matter can release very large amounts of energy.