Nuclear Review
advertisement

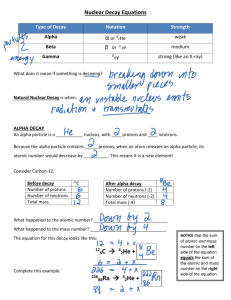
Chemistry Name_________________________________________________ 1. The atomic number of uranium is 92. When a nucleus of U-235 absorbs a neutron it quickly splits into two daughter nuclei and releases several particles. a) What type of nuclear reaction is this? b) How many protons are there in the nucleus described above? c) What isotope of uranium is formed when the neutron is absorbed? d) What subatomic particles are released when the uranium splits into the daughter nuclei? 2. Write equations for the following: a) Beta decay of Th-234 b) Alpha decay of Th-230 c) Beta decay of Bi-213 d) Alpha decay of Po-216 3. Identify which type of radiation is described by each statement below: a) Has the largest mass b) Causes the most ionization c) Is identical to a helium nucleus d) Has zero electrical charge e) Is an electron travelling at a high speed 4. During alpha decay, how many protons and neutrons does a nucleus lose? 5. What is a transmutation? 6. What happens to the number of protons and neutrons in the nucleus during a beta decay? 7. It takes 10 years for the amount of a radioisotope to decrease to 1/16 its original amount. Calculate the half-life of the material. 8. How would you expect rubidium to react with water? Write the equation for the reaction. 9. An element has an atomic radius of 0.099 nm and an ionic radius of 0.181 nm. Is it more likely to be an atom from group 2 or group 17? Explain your answer. 10. An atom has a radius of 181 Å. Convert the radius to meters. 11. Which would you expect to be more reactive, Be or Ca? Why? 12. Explain why francium has the largest radius and in group 1 and explain why it has the largest radius in period 7