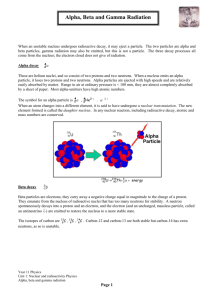
Nuclear Decay Notes
advertisement
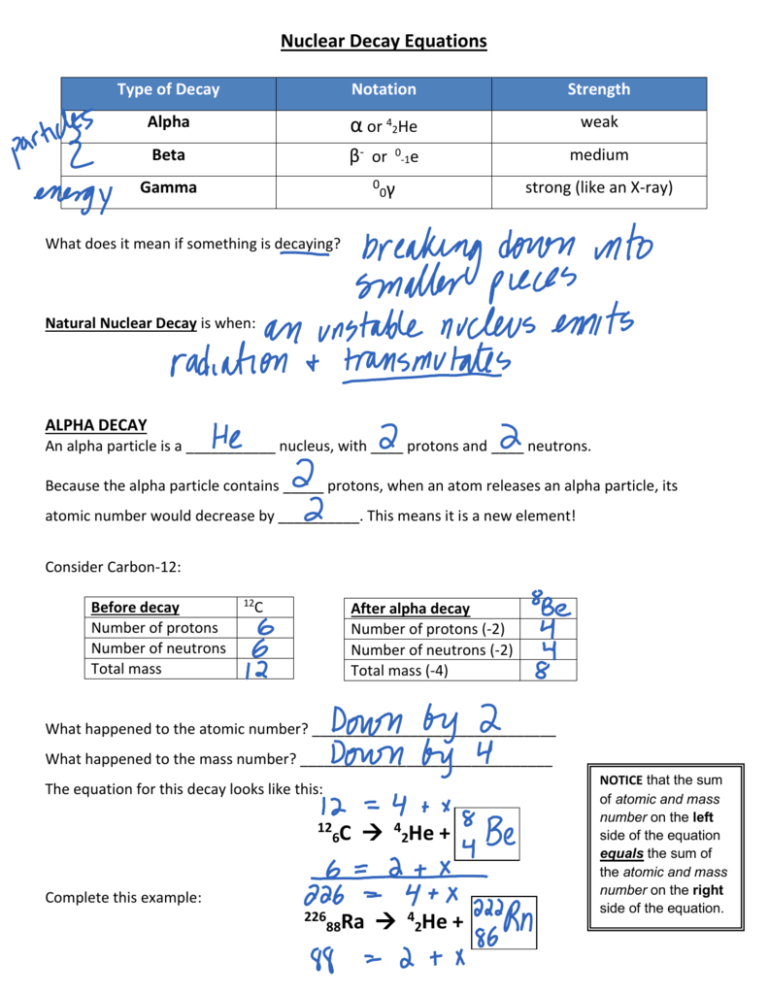
Nuclear Decay Equations Type of Decay Notation Strength Alpha α or 42He weak Beta β- or 0 Gamma medium 0 -1e strong (like an X-ray) 0γ What does it mean if something is decaying? Natural Nuclear Decay is when: ALPHA DECAY An alpha particle is a ___________ nucleus, with ____ protons and ____ neutrons. Because the alpha particle contains _____ protons, when an atom releases an alpha particle, its atomic number would decrease by __________. This means it is a new element! Consider Carbon-12: Before decay Number of protons Number of neutrons Total mass 12 C After alpha decay Number of protons (-2) Number of neutrons (-2) Total mass (-4) What happened to the atomic number? ______________________________ What happened to the mass number? _______________________________ The equation for this decay looks like this: 12 6C 4 2He + Complete this example: 226 88Ra 4 2He + NOTICE that the sum of atomic and mass number on the left side of the equation equals the sum of the atomic and mass number on the right side of the equation. BETA DECAY A beta particle is equivalent to an _______________. (A neutron splits, leaving a proton behind). n p + eWhen an atom releases a beta particle, its atomic number would increase by __________. This means it is a new element! Consider Carbon-14: Before decay Number of protons Number of neutrons Total mass 14 C After beta decay Number of protons (+1) Number of neutrons (-1) Total mass (+0) What happened to the atomic number? ______________________________ What happened to the mass number? _______________________________ The equation for this decay looks like this: 14 6C 0 -1e + Complete this example: 131 53I 0 -1e + GAMMA DECAY A gamma ray is ______________, (not a particle) resulting from an energy change within the nucleus. It has _____ mass or charge. Gamma radiation doesn’t change the state of the nucleus, it just carries some energy away. Gamma rays are emitted along with alpha or beta particles.