9th Grade Physical Science #2A Unit 2. Get a Charge Out of Matter
advertisement

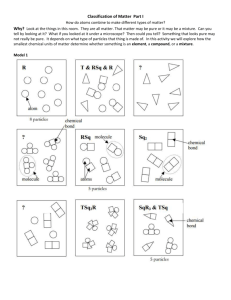
9th Grade Physical Science Unit 2. Get a Charge Out of Matter Activity #2A: Classifying Matter by Its Composition #2A To Begin In activity #1 you thought about what might be different between salt water and pure water. You reasoned what it means to dissolve sugar into water. In this activity, you will begin to classify particles of matter (such as salt, water and sugar) based on their sub-microscopic composition. First, please complete the reading, A Short History of Chemistry. As you read, highlight key passages and make notes as part of your individual reading strategies. Then you will come together as a group to agree on the central idea of the text and use key details to support your choice. Learning Goals for this activity Students will … • determine the central idea of a text and support it with key details • use of model to to compare and contrast the terms: element, compound, molecule, atom, pure substance, mixture, homogeneous and heterogeneous. Process & Procedure 1. a. As a team, identify the central idea of the text, A Short History of Chemistry, as it relates to matter and to human technology. Identify 5-7 key details that support the central idea of that reading. Each oval should contain a descriptive phrase about a one key detail. Central idea: b. Add one of your team’s key details to the active board at the front of the room . Be sure that the your choice of detail has not already been used. 2. You will work with a partner to build models of different types of molecular particles. Molecular particles show the sub-microscopic structure of the particle…shows the # and type of atoms that make up the molecular particle. 3. Discuss each term and its definition, listed below, with your partner. Build a model of a molecular particle that matches each definition. Sketch your model and write a note that explains the structure of that molecular particle. Use shading or colored pencils to sketch the different atoms involved. Be sure to show your models to your teacher before you put them back. Single Particles Type of Matter Atom Molecule Element Definition Sketch with Annotation (description) The smallest particle of an element. Can combine with other atoms. choose an atom found in water The smallest particle of a pure substance that still possesses the properties of the substance (could be an element or a compound) choose a molecule of water A pure substance made of only one type of atom. choose a valuable element Multiple Particles Compound A pure substance that is composed of molecules made of more than one kind of element (atom). your choice: make three particles of it Pure A sample of matter that consists of Substance only one kind of matter (could be compound or element) your choice: make several particles; identify your pure substance as an element or compound Mixture A sample of matter consisting of two or more pure substances. The pure substances in a mixture could be physically separated from each other. homogenous heterogenous •Homogenous- evenly mixed throughout •Heterogenous- unevenly mixed Stop and Think 1. The prefix di means two. A diatomic molecule is a molecule made of two atoms (either same atoms or different atoms. Describe and sketch a particle model that would represent a diatomic element (same atoms) and a diatomic compound (different atoms). Diatomic element Diatomic compound 2. Describe an actual example found in your daily life of a pure substance, a homogenous mixture and a heterogeneous mixture.