Binding Energy and Nuclear Forces
advertisement
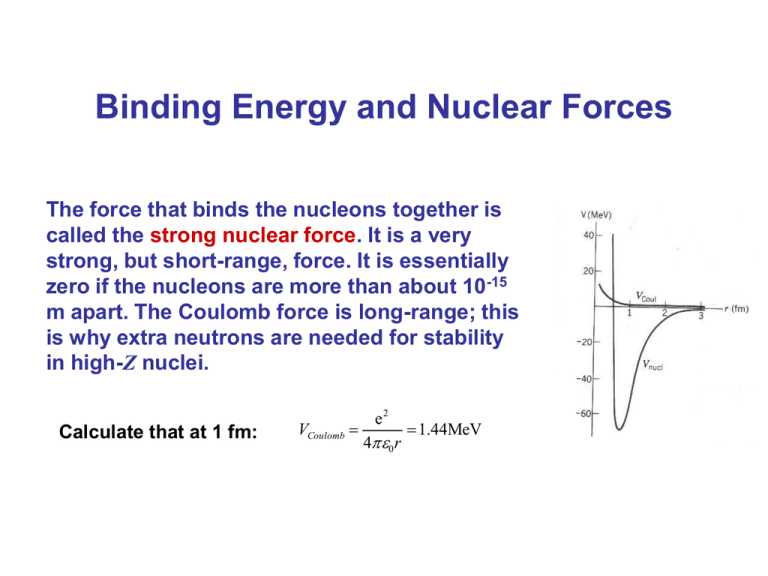
Binding Energy and Nuclear Forces The force that binds the nucleons together is called the strong nuclear force. It is a very strong, but short-range, force. It is essentially zero if the nucleons are more than about 10-15 m apart. The Coulomb force is long-range; this is why extra neutrons are needed for stability in high-Z nuclei. Calculate that at 1 fm: VCoulomb e2 40 r 1.44MeV Structure and Properties of the Nucleus Nuclei: protons and neutrons. Proton has positive charge; mn = 1.67262 x 10-27 kg Neutron is electrically neutral; mn = 1.67493 x 10-27 kg Number of protons: atomic number, Z Number of nucleons: atomic mass number, A Neutron number: N = A - Z Symbol: Isotopes mp me 1836.152 672 61 (85) No theory Extreme density Because of wave–particle duality, the size of the nucleus is somewhat fuzzy. Measurements of high-energy electron scattering yield: Masses scale: carbon-12 atom, 12 u u is a unified atomic mass unit. Magnetic moments For a classical point particle L gLB g L 1 Define nuclear magnetic moments (unit = the nuclear magneton) N e 2M p For a relativistic spin (point) S S g S B Proton magnetic moment gS 2 p 2.7928 N Neutron magnetic moment n 1.9135 N Protons and neutrons are not point particles Binding Energy and Einsteins E=mc2 Binding energy affects mass of composite particle mH-atom < mproton + melectron mH-atom(n=1 state) < mH-atom(n=2 state) Eb atom M nucleus Zme M atom c 2 (mind the signs !) These effects are much larger in the realm of nuclei than in that of electromagnetism Eb nucleus ZM p NM n M nucleus c 2 Binding Energy and Nuclear Forces Eb nucleus ZM p NM n M nucleus c 2 The total mass of a stable nucleus is always less than the sum of the masses of its separate protons and neutrons. Eb nucleus ZM p NM n Zme M atom c 2 Here we neglect of the binding energy inside the atom (eV). Eb A X ZM 1H NM n M A Xc2 Binding of nucleus Mass of neutral atom This can be used to calculate Eb/A: binding energy per nucleus Binding Energy and Nuclear Forces From observation: binding energy per nucleon: Eb/A Note: a is point of relative stability Binding Energy and Nuclear Forces From observation: The higher the binding energy per nucleon, the more stable the nucleus. More massive nuclei require extra neutrons to overcome the Coulomb repulsion of the protons in order to be stable. Addition The semi-empirical mass formula Von Weizsäcker Liquid drop model Eb A X a1 A a2 A2 / 3 a3 A1/ 3 a4 Z2 A / 2 Z 2 A 5 Note: R ~ A1 / 3 Volume term 3 ~R ~A a1 15.76Mev a2 17.81Mev Good fit for: a3 0.7105Mev a4 94.80Mev a5 39Mev Surface correction: on the outer surface (4R2) the binding is less, because There are no particles to contribute ~ R 2 ~ A2 / 3 Addition The semi-empirical mass formula Von Weizsäcker Liquid drop model Vrep Eb A X a1 A a2 A2 / 3 a3 A1/ 3 a4 Z2 3 Ze 2 5 40 R a5 A3 / 4 0 Coulomb energy stored in a uniform solid sphere of charge Ze and radius R (negative binding) A / 2 Z 2 ~ Z2 A1 / 3 A ee oo eo oe “pairing energy” (not further discussed) 5 Addition Protons and Neutrons in the Fermi-gas model a4 A / 2 Z 2 A Both protons and neutrons cannot fill the lowest “orbitals” because the have to follow the Pauli exclusion principle “ Symmetry Energy” preference for ZN Based on this concept: the “nuclear shell model” "for the discovery concerning nuclear shell structure" Maria Goeppert-Mayer Addition Isobars and the mass equation Mass of nucleus: M 2 Z2 A / 2 Z 2 2/3 X ZM H ( A Z ) M n a1 A a2 A a3 1 / 3 a4 5 / c A A A Isobar: 1 Z A / 2 M 2 Z c M 1 H M n c 2 2a3 1/ 3 a4 Z A A Minimum charge Z: A a4 M 1 H M n c 2 ZA 2 a4 a3 A2 / 3 Valley of stability (even-odd separate due to 5 term) Unstable elements t = 2 x 106 y 99Tc t = 2 x 105 y 97Tc t = 18 y 146Pm t = 5.5 y 145Pm Stable/Meta-stable elements 126Te stable, 18.95 % 128Te t > 5 x 1024 y 130Te t = 2.5 x 1021 y t = 1.6 x 105 y 235U t = 7 x 108 y 238U t = 4.5 x 109 y 233U Why do nuclei decay radioactivity