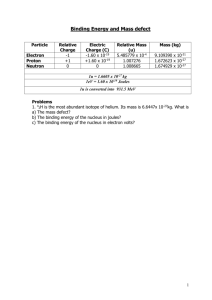
The binding energy gives us a quantitative measure of the degree of
advertisement

When a nucleus forms from the individual particles a small percent of the mass of each of these protons and neutrons appears to disappear. This “missing” mass is known as the mass defect. In actuality this mass defect is converted to energy according to the equation E= mc2 and this energy is release as infrared radiation (heat) visible radiation (light) ultraviolet radiation, x-ray radiation, and gamma radiation. This energy is called the binding energy because it provides the force which holds or binds all of the nucleons (the positively charged protons and the neutrally charged neutrons) together in a very small area. The binding energy can be defined as the amount of energy that is released when a nucleus is formed from its subatomic particles. Conversely, the binding energy is the amount of energy that must be replaceded to break the nucleus apart. The greater the amount of binding energy per nucleon the more stable the nucleus is. To calculate the binding energy 1--find the total mass of the individual particles. mp=1.007825 amu mn=1.008665 amu 2--calculate the mass defect by subtracting the mass of the atom from the mass of the particles. 3--convert the mass defect into the binding energy it would provide using the equation E = mc2. (c = 1.22 x 10-5) 4—Divide the binding energy by the number of nucleons.