Physiology Ch. 40 p495-504 [4-25
advertisement

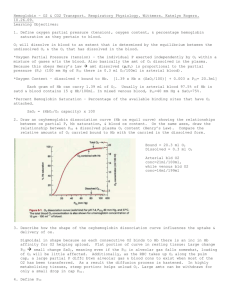
Physiology Ch. 40 p495-504 Transport of O2 and CO2 in Blood and Tissue Fluids -Once oxygen has diffused into pulmonary blood, it is carried to tissues almost entirely on hemoglobin. -Hgb can transport 30-100x as much O2 as dissolved O2 in H2O alone Transport of O2 from Lungs to Body Tissues – in tissues of the body, there is a higher PO2 in the capillaries than the tissues, causing O2 to diffuse into the surrounding cells. -When O2 is metabolized by cells, resulting PCO2 in tissues grows, causing it to diffuse into capillaries -CO2 in blood will flow to the lung and diffuse out of the blood into alveoli Diffusion of O2 from Alveoli to Pulmonary Capillary Blood – PO2 of gaseous O2 in alveolus averages 104mm Hg, PO2 of venous blood entering pulmonary capillary is 40mmHg. Initial pressure difference causes O2 to diffuse is 64mmHg. -Rapid rise in PO2 as blood passes through capillary because more and more O2 diffuses in Uptake of O2 by Pulmonary Blood During Exercise – during exercise, body may require up to 20x normal amount of O2. -increased cardiac output reduces time in capillaries by 2. -Safety Factor for diffusion of O2 through pulmonary membrane causes blood to still be saturated with O2 by the time it leaves capillaries. -diffusion capacity for O2 increases 3-fold during exercise and results mainly from increased surface area of capillaries participating in diffusion -under non-exercising conditions, blood becomes saturated with O2 by the time it has passed through 1/3 of pulmonary capillary, but oxygenation doesn’t take place in latter 2/3ds, so even during exercise with blood traveling faster, it still gets full oxygenation Transport of O2 in Arterial Blood – 98% of blood entering left atrium passes through alveolar capillaries. 2% of the blood goes through bronchial circulation is not exposed to lung air and is called shunt flow. Combining with pulmonary circulation blood, this mixture is called venous admixture of blood. Diffusion of O2 from Peripheral Capillaries into Tissue Fluid – when arterial blood reaches tissues, PO2 in capillaries is 95mmHg whereas PO2 in interstitial fluid is 40mmHg, so pressure difference causes O2 to diffuse from blood to tissues very rapidly Effect of rate of Blood Flow on Interstitial Fluid PO2 – if blood flow through particular tissue is increased, greater quantities of O2 are transported to tissue and tissue PO2 becomes higher -upper limit to PO2 is 95mmHg because that is what it is in arterial blood Effect of Rate of Tissue Metabolism on Interstitial Fluid PO2 – if cells use more O2 for metabolism than normally, this reduces interstitial PO2. -PO2 is determined by rate of O2 transport to tissues and rate of O2 metabolism by tissues Diffusion of O2 from Peripheral Capillaries to Tissue Cells – intracellular PO2 in peripheral tissues is lower than PO2 in capillaries because tissues always metabolizing O2 Diffusion of CO2 from Peripheral Cells into Capillaries – all O2 becomes CO2 in cells, which increases PCO2. This causes diffusion from cells into capillaries that carry CO2 to the lungs. -at each point in gas transport, CO2 diffuses opposite to O2, but CO2 diffuses 20x MORE RAPIDLY! -Pressures of CO2 in each of the following locations 1. Intracellular PCO2 = 46mmHg; Interstitial PCO2 = 45 mmHg 2. Artery PCO2 = 40 mmHg; PCO2 venous blood = 45 mmHg 3. PCO2 blood in pulmonary capillaries = 45 mmHg; PCO2 alveolar air = 40 mmHg Effect of Rate of Tissue Metabolism and Tissue Blood Flow on Interstitial PCO2 – a decrease in blood flow from normal to ¼ of normal increases peripheral tissue PCO2 from 45 mmHg – 60 mmHg -Increasing blood flow to 6x normal drops the PCO2 to 41mmHg -10x increase in tissue metabolic rate increases interstitial PCO2 at all rates of blood flow Role of Hemoglobin in O2 Transport – 97% of all O2 in blood carried by hemoglobin -O2 combines loosely and reversibly with heme of hemoglobin -When PO2 is high (in pulmonary capillaries), O2 binds hemoglobin; when low, O2 released from heme Oxygen-hemoglobin dissociation curve – this curve shows progressive increase in % hemoglobin bound to O2 with increasing PO2, called %Saturation of Hemoglobin -usual saturation of hemoglobin in arterial blood is 97% -Venous hemoglobin averages 75% saturation -total amount O2 that can combine with 15g hemoglobin in 100mL blood at 100% saturation is 20mL O2 -amount of O2 in capillaries as blood passes tissues reduces from 19.4mL to 14.4 mL; 5mL of O2 is transported from lungs to tissues with each 100mL of blood -During exercise, muscle cells use O2 at a rapid rate, causing PO2 to drop to ~15mmHg, which means 4.4mL of O2 is bound to hemoglobin, so 15mL of O2 is delivered to tissues by 100mL of blood (3x more) -coupled with 6-7x cardiac output, O2 transport in each volume of blood can increase 20-fold Utilization Coefficient – % of blood that gives up O2 as it passes through tissue capillaries (norm = 25%) -during strenuous exercise, value goes up to 75-85% Effect of Hemoglobin to Buffer Tissue PO2 – in addition to carrying O2, hemoglobin acts as a tissue oxygen buffer, which stabilizes O2 pressure in the tissues -tissues require 5mL O2/100mL blood, meaning PO2 must fall to 40mmHg; hemoglobin normally sets an upper limit on O2 pressure in tissues at 40mmHg -During heavy exercise, hemoglobin delivers O2 at a pressure held tightly between 15 and 40mmHg -If atmospheric O2 concentration changes, hemoglobin still maintains constant tissue PO2 -Tissue PO2 hardly changes even with drastic fall in alveolar PO2 from 104-60mmHg at high altitudes -When Alveolar PO2 rises as high as 500mmHg, max O2 saturation of hemoglobin cannot rise above 100%, and only a small amount of additional O2 dissolves -Hemoglobin therefore buffers the O2 concentration regardless of atmospheric oxygen Factors That Shift O2-hemoglobin Dissociation Curve – slight acidity (<pH) moves dissociation durve to the right, whereas slight basicity shifts the curve to the left. Besides pH, 3 things can alter curve: 1. Increased temperature – during exercise, several things shift curve to the right to deliver more O2 to muscles, which release more CO2 and more H+ into blood. Temp. of muscle fibers increases 2-3 degrees Celcius to deliver O2 even more. 2. Increased CO2 Concentration – increases release of O2 from blood in tissues and enhances oxygenation of blood in the lungs, called the Bohr effect. a. As blood passes tissues, CO2 diffuses in to blood, increasing PCO2, which raises H2CO3 and the H+ concentration, which shifts O2-heme dissociation curve to the right b. Opposite occurs in the lungs, where CO2 diffuses into alveoli which reduces PCO2 in blood and decreases H+ in blood, shifting dissociation curve to the left 3. Increased 2, 3-biphosphoglycerate (BPG) – normal BPG in blood keeps O2-heme curve shifted to the right all the time. In hypoxia > few hours, BPG increases shifting curve more to the right, therefore BPG can be important to adaptation to hypoxia Metabolic Use of O2 by the Cells – only a minute level of O2 is required for normal cellular respiration. Limiting factor is not O2 but rather adenosine diphosphate (ADP) in the cells. -When ADP concentration is altered, rate of O2 usage changes in proportion to change in ADP concentration -Under normal operating conditions, rate of O2 usage by cells is controlled ultimately by rate of energy expenditure by cells, by rate at which ADP is formed from ATP Effect of Diffusion Distance from Capillary to Cell on Oxygen Usage – most tissues less than 50um away from capillaries, but sometimes cells can be further away. Intracellular PO2 here becomes lower than critical level required for metabolism; it is said these cells are Diffusion Limited, where use is not determined by ADP; does not occur unless pathologic state Effect of Blood Flow on Metabolic Use of O2 – amount of O2 dependent on quantity of O2 transported and the rate of blood flow. If flow falls to 0, amount of available O2 falls to 0. -If tissues show lack of O2 due to reduced blood flow (<1mmHg O2), it is said to be blood flow limited. Transport of O2 in Dissolved State – at normal arterial PO2, 0.29mL O2 dissolved/100mL blood, therefore amount of O2 transported to tissues in dissolved state is slight (3% of total) -during strenuous exercise, dissolved O2 transported to tissues falls to 1.5% -if person breathes O2 at high alveolar PO2 levels, amount transported can become greater, can cause O2 poisoning and brain convulsions -Carbon Monoxide can combine with hemoglobin same as O2 but with 250x as much tenacity, which can be lethal for a human -O2 in blood is reduced during CO poisoning, but PO2 stays the same, dangerous because blood is red and no symptoms of hypoxemia -since PO2 not reduced, normal feedback to stimulate respiration by low PO2 is not present, so person can become disoriented and unconscious due to low O2 in brain Transport of CO2 in the Blood – Under normal conditions, 4mL CO2 transported from tissues to lungs per 100mL of blood -CO2 diffuses out of tissue cells in dissolved CO2 form, of which a small % is transported to the lungs (7% of all CO2 or 0.3mL/100mL blood) -Dissolved CO2 reacts with H2O to form carbonic acid (H2CO3) in the presence of carbonic anhydrase which catalyzes the reaction 5000-fold. Allows most of CO2 to be converted in fraction of a second -Carbonic acid in RBCs dissociates into H+ and HCO3. H+ combines with hemoglobin and HCO3 diffuse from RBCs into plasma in place of Chloride ions. Takes place with the Bicarbonate-chloride carrier protein in RBC cell membrane -chloride of venous RBC is greater than arterial RBC, called Chloride Shift. -CO2 can react directly with amine radicals of hemoglobin molecule to form carbaminohemoglobin (CO2Hgb). Reversible reaction that occurs with a loose bond, so CO2 easily released into alveoli where PCO2 is lower than capillaries -amount of CO2 transported from peripheral tissues to lungs by carbaminohemoglobin is 30% of total transported, or 1.5mL of CO2 per 100mL of blood CO2 Dissociation Curve – depicts dependence of blood CO2 in all its forms on PCO2, ranges between 4045mmHg for normal. Normal concentration in all forms is 50 volumes percent, but 4 volumes is exchanged during normal transport of CO2 from tissues to lungs. -When O2 binds hemoglobin, CO2 is released from the blood, called the Haldane Effect. Results from fact that combining O2 with hemoglobin in lungs causes hemoglobin to become stronger acid, which displaces CO2 from blood and into alveoli in 2 ways: 1. Acidic hemoglobin has less tendency to combine with CO2, displacing CO2 from blood 2. Increased acidity of hemoglobin causes it to release extra H+ ions which bind to HCO3 to form H2CO3 which dissociates to form H2O and CO2, and CO2 is released from blood into alveoli -On entering lungs, PCO2 falls to 40mmHg and PO2 rises to 100mmHg. If CO2 dissociation curve did not shift because of Haldane Effect, CO2 of blood would fall to 50 volumes percent, which would be a loss of only 2 volumes % of CO2. However, increase in PO2 in lungs lowers CO2 dissociation curve so that the CO2 content falls to 48 volumes %. -Haldane effect doubles amount of CO2 released from blood in the lungs and approximately doubles pickup of CO2 in lung tissues Change in Blood Acidity during CO2 Transport – H2CO3 formed when CO2 enters blood in peripheral tissues decreases blood pH, but buffers in the blood prevent H+ from rising rapidly. -Arterial pH is 7.41, venous pH is around 7.37 -Exercise can cause severe acidosis with a decrease in pH Respiratory Exchange Ratio – 82% as much CO2 is expired from lungs as oxygen is taken up. (5mL O2 taken up and 4mL of CO2 expired). -Ratio of CO2 otuput to oxygen uptake is called respiratory exchange ratio: R = Rate of CO2 Output/Rate of O2 Uptake -value of R changes under different metabolic conditions -if person using carbs, exchange ratio rises to 1.00 -if person uses fats for energy, ratio is as low as 0.7 -more O2 combines with fats than with carbs for metabolic activity