Phys chapter 40 [10-2
advertisement

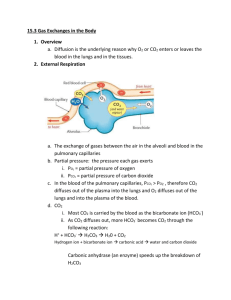
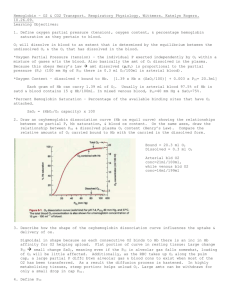
Chapter 40 Phys Transport of Oxygen from Lungs to Body Tissues Oxygen and carbon dioxide transfer between systemic capillaries and body tissues is same as gas exchange in lungs, but there is higher PCO2 and lower PO2 in body tissues Because of great safety factor for diffusion of oxygen through pulmonary membrane, blood becomes almost saturated with oxygen by the time it leaves the pulmonary capillaries, even during strenuous exercise when the body requires so much more oxygen and blood flows through capillaries much faster o Surface area is increased because of opening of accessory capillaries o More nearly ideal ventilation-perfusion ratio in upper part of lungs o Under normal resting conditions, blood becomes nearly saturated with oxygen before it makes 1/3 of the transit through the pulmonary capillaries, and very little oxygen enters the blood during the last 2/3 of transit 98% of blood that enters left atrium has been fully oxygenated from alveolar capillaries (PO2 = 104 mm Hg), and the other 2% is from bronchial circulation (shunt flow, PO2 = 40 mm Hg) o Because of the huge percentage of oxygenated blood, blood in systemic circulation still has a much higher PO2 than tissues (95 mm Hg), and diffusion still occurs If cells use more oxygen for metabolism than normal, this reduces interstitial fluid PO2 Oxygen is always being used by cells, so intracellular PO2 in peripheral tissue is always lower than PO2 in peripheral capillaries o Often, there is considerable distance between capillaries and cells, so normal intracellular PO2 ranges from 5 mm Hg to 40 mm Hg o Only 1-3 mm Hg is needed for full support of chemical processes that use oxygen in the cell so average of 23 mm Hg is more than sufficient (large safety factor) When oxygen is used by cells, virtually all of it becomes CO2, increasing intracellular PCO2 o Large difference between intracellular PCO2 and capillary PCO2, CO2 diffuses from cells to tissue capillaries and is then carried by blood to lungs o CO2 diffuses in exact opposite direction as O2 along all points, but CO2 can diffuse about 20x as rapidly o Because of above, PCO2 differences required are much less (46 mm Hg in intracellular, 45 mm Hg in interstitial, 40 mm Hg in arterial blood, and 45 mm Hg in venous blood) o PCO2 level of pulmonary circulation has reached the arterial level before it traverses 1/3 of its course through pulmonary capillaries (same as O2) Tissue capillary blood flow and tissue metabolism affect PCO2 in ways exactly opposite to effect on tissue PO2 (decrease in blood flow increases peripheral tissue PCO2, etc.) Normally, 97% of O2 transported in blood is carried in chemical combination with Hgb in RBCs, and remaining 3% is transported in dissolved state in water of plasma and blood cells o When PO2 is high, as in pulmonary capillaries, O2 binds to heme of hemoglobin, but when PO2 is low, as in tissue capillaries, oxygen is released from heme o In normal systemic arterial blood, oxygen saturation of heme averages 97% o In venous blood returning from peripheral tissues, saturation of heme averages 75% Utilization coefficient – percentage of blood that gives up its oxygen as it passes through tissue capillaries (normal value is 25%, but during strenuous exercise, can increase to 75-85%) o In local tissue areas where blood flow is extremely slow or metabolic rate is very high, utilization coefficients approaching 100% have been recorded Main function of hemoglobin is as a tissue oxygen buffer system, stabilizing oxygen pressure in tissues o If tissue PO2 rises above a certain point, the amount of oxygen needed by tissues would not be released from hemoglobin, so hemoglobin sets an upper limit on tissue PO2 o During heavy exercise, extra amounts of oxygen must be delivered from hemoglobin to tissues – can be achieved with little further decrease in tissue PO2 because of steep slope of oxygen dissociation curve and increase in tissue blood flow caused by decreased PO2 (very small fall in PO2 causes large amounts of extra oxygen to be released from hemoglobin) When atmospheric oxygen concentration changes markedly (as could be seen by change in altitude), buffer effect of hemoglobin maintains almost constant tissue PO2 o PO2 measurements of venous and arterial blood can drop by small amounts, but same amount of O2 is delivered to and used by tissues (40% reduction in alveolar PO2 only causes about 5 mm Hg drop in venous blood PO2) o Hemoglobin is normally 97% saturated, so even in very oxygen rich environment, it can’t rise above 100%, so it stays close to the same Factors that affect oxygen dissociation curve o Shift to right caused by increased hydrogen ions, increased CO2, increased temperature, and increased BPG (2,3-biphosphoglycerate – metabolically important phosphate compound in blood in different concentrations under different metabolic conditions) o Opposite conditions will shift curve to left o Shift of oxygen-hemoglobin dissociation curve in response to changes in blood CO2 and H+ is caused by Bohr effect – as blood passes through tissues, CO2 diffuses from tissue cells into blood, increasing blood PCO2, which raises blood H+ (because of carbonic acid), so increasing H+ would shift equilibrium so oxygen is forced away from hemoglobin, and increased amounts of oxygen would be delivered to tissues o Normal BPG in blood keeps oxygen-hemoglobin dissociation curve shifted slightly to right at all times, but in hypoxic conditions that last longer than a few hours, quantity of BPG in blood increases considerably, shifting oxygen-hemoglobin dissociation curve even farther to right, causing oxygen to be released to tissues (adaptation to hypoxia) o During exercise, dissociation curve shifted down and right because exercising muscles release large quantities of CO2 and muscles release acids, increasing H+ – also, temperature of muscles can rise 2-3o C, which can increase oxygen delivery to muscle fibers even more (in lungs, shift occurs in opposite direction, allowing pickup of extra amounts of oxygen from alveoli) Respiratory enzyme systems of cells geared so that when cellular PO2 is more than 1 mm Hg, oxygen availability is no longer a limiting factor in rates of chemical reactions – main limiting factor is concentration of ADP in cells (when ADP concentration is altered, rate of oxygen usage changes in proportion to change in ADP concentration) o Increasing concentration of ADP (from used ATP) increases metabolic usage of oxygen as it combines with various cell nutrients, releasing energy that reconverts the ADP back to ATP o Under normal operating conditions, rate of oxygen usage by cells is controlled ultimately by rate of energy expenditure within cells (rate at which ADP is formed from ATP) Diffusion limited oxygen usage in cells (when oxygen diffusion becomes so low that intracellular PO2 falls below critical level required to maintain maximal intracellular metabolism) almost never occurs except in pathological conditions (or very rare instances when cells are more than 50 micrometers away from a capillary) Total amount of oxygen available for use by tissue is determined by quantity of oxygen that can be transported to tissue and rate of blood flow o If blood flow falls and causes tissue PO2 to fall below critical value, rate of tissue usage of oxygen is blood flow limited During strenuous exercise, when hemoglobin release of oxygen to tissues increases threefold, relative quantity of oxygen transported in dissolved state falls to ½ the normal percentage If a person breathes oxygen at very high alveolar PO2 levels, amount transported in dissolved state can become much greater, sometimes so much that serious excess of oxygen occurs in tissues (oxygen poisoning), leading to brain convulsions and death Carbon monoxide binds to hemoglobin at same point as oxygen, so it can displace oxygen, decreasing the oxygen-carrying capacity of blood – binds with about 250x the affinity as oxygen, so even a PCO as little as 0.6 mm Hg (less than one part per thousand) in air can be lethal o Even though oxygen content of blood is greatly reduced, PO2 may be normal, so there may be no signs of hypoxemia and feedback mechanism that usually stimulates increased respiration rate in response to lack of oxygen is absent o Because brain is one of first organs affected by lack of oxygen, person may become disoriented or unconscious before becoming aware of danger o Patients with CO poisoning can be treated with pure oxygen because oxygen at high alveolar pressure can displace CO – patient can also benefit from simultaneous administration of 5% CO2 because this strongly stimulates the respiratory center, which increases alveolar ventilation and reduces alveolar CO Transport of Carbon Dioxide in Blood Even in most abnormal conditions, CO2 can usually be transported in far greater quantities than O2, but amount of CO2 in blood affects acid-base balance of body fluids CO2 diffuses out of tissue cells in dissolved molecular CO2 form; on entering tissue capillaries, CO2 initiates almost instantaneous physical and chemical reactions essential for CO2 transport (can be dissolved CO2, can combine with Hgb, or can be converted to H2CO3 by carbonic anhydrase) o 7% of transported CO2 stays dissolved, 20% combines with hemoglobin and other plasma proteins, and 70% is by conversion to carbonic acid (and dissociated bicarbonate) Transport of CO2 in form of bicarbonate ion – dissolved CO2 reacts with H2O to form carbonic acid because of carbonic anhydrase – because of enzyme, this reaction reaches almost complete equilibrium in a small fraction of a second o H2CO3 dissociates into H+ and HCO3o Most of H+ combine with hemoglobin in RBCs because hemoglobin is powerful acid-base buffer, and much of HCO3- diffuses from RBC into plasma and Cl- diffuses into RBCs to balance charge (made possible by bicarbonate-chloride carrier protein in RBC membrane) o Higher chloride content of venous RBCs than arterial RBCs called chloride shift CO2 reacts directly with amine radicals of hemoglobin to form carbaminohemoglobin – reversible reaction with a loose bond so CO2 easily released into alveoli Small amount of CO2 reacts with plasma proteins in tissue capillaries by carbamino combination Carbon dioxide dissociation curve – depicts dependence of total blood CO2 in all its forms on PCO2 o PCO2 held in fairly narrow range in blood and very little CO2 is exchanged between tissues and lungs o Binding of oxygen with hemoglobin can displace CO2 from blood (Haldane effect) – far more significant in affecting blood gas levels than Bohr effect Results from fact that combination of O2 with hemoglobin in lungs causes hemoglobin to become a stronger acid, displacing CO2 from blood into alveoli because More highly acidic hemoglobin has less tendency to combine with CO2, thus displacing much of CO2 in carbaminohemoglobin form Increased acidity of hemoglobin causes it to release excess of H+, which bind with bicarbonate ions to form carbonic acid, which dissociates into water and CO2 (which is released from blood to alveoli) o If CO2 dissociation curve did not shit because of Haldane effect, CO2 content of blood would only fall 2 volumes percent, but increase in PO2 in lungs lowers CO2 dissociation curve so CO2 content falls 4 volumes percent – Haldane effect doubles amount of CO2 released from blood in lungs and approximately doubles pickup of CO2 in tissues Arrow represents Haldane effect Reaction of carbonic acid formed when CO2 enters blood with acid-base buffers of blood prevents H+ concentration from rising greatly (and pH from falling greatly) – arterial blood has pH of about 7.41 and venous blood has pH of about 7.37 o In heavy exercise or other conditions of high metabolic activity, or when blood flow through tissues is sluggish, decrease in pH in tissue blood (and tissues themselves) can be as much as 0.50, causing significant tissue acidosis Respiratory Exchange Ratio Respiratory exchange ratio (R) – ratio of carbon dioxide output to oxygen uptake When a person is using exclusively carbohydrates for body metabolism, R rises to 1, and when person is using exclusively fats for metabolic energy, R falls to 0.7 o When oxygen is metabolized with carbohydrates, one molecule of CO2 is formed for each molecule of O2 consumed o When oxygen reacts with fats, large share of O2 combines with hydrogen atoms from fats to form water instead of CO2 For a person on a normal diet, average R is about 0.825