solutions
advertisement

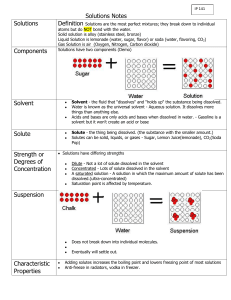
Name_____________________________ Date_______________ Period_________ SOLUTIONS Fill in the blank (use your notes from the presentation we have covered in class so far): Dissolving is a _____________________change because no new substances are produced. A solution is a ______________________ mixture of two or more substances in a ______________ phase. A solution is made of a ________________ and a ____________________. A _________________ is the substance that is dissolved in the ___________________. The solute is present in the ______________________amount and the solvent is present in the _______________amount. The ______________________determines the state of the solution. An example of a solution with a gas solute and a gas solvent is _______________. Rubbing alcohol is an example of a solution with a ________________ solute and a ____________________solvent. An alloy is a mixture of two _________________. In order to dissolve a substance, three things can be done to speed the process: ____________________, increasing the _____________________, and ________________the solute to make it smaller. ____________________is the maximum amount of solute that will dissolve in a given amount of solvent at a specified temperature and pressure. When a solute can be completely dissolved in the solvent, it is said to be _____________________. If the solute cannot be dissolved in the solvent, then it is said to be _______________________. When two liquids completely mix, they are said to be ___________________. If two liquids do not mix, they are said to be ____________________. Conservation of Matter Balance the following chemical equations: 1 . _____ H2 + _____ O2 _____ H2O 2. _____ CO2 + _____ H2O _____ C6H12O6 + _____ O2 3. _____ SiCl4 + _____ H2O _____ H4SiO4 + _____ HCl 4. _____ Na + _____ H2O _____ NaOH + _____ H2 5. _____ C10H16 + _____ Cl2 _____ C + _____ HCl 6. _____ Fe2(SO4)3 + _____ KOH 7. _____ FeS2 + _____ O2 8. _____ Fe2O3 + _____ H2 9. ____ AlBr3 + ____ K2SO4 10. ____ H2SO4 + ____ NaNO2 _____ K2SO4 + _____ Fe(OH)3 _____ Fe2O3 + _____ SO2 _____ Fe + _____ H2O ____ KBr + ____ Al2(SO4)3 ____ HNO2 + ____ Na2SO4