Solubility: the mass of the solute that is dissolved in a certain amount
advertisement

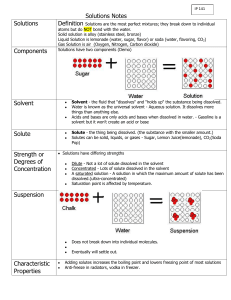
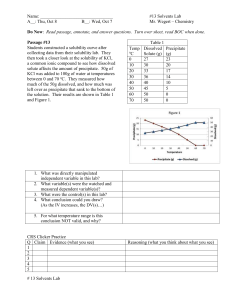
Solubility: the mass of the solute that is dissolved in a certain amount of solvent to form a saturated solution at a specific temperature. FACTORS AFFECTING SOLUBILITY 1. type of solute: some solutes have more attraction towards the solvent particles than other solutes. some solute particles are larger than other solutes which means less is needed to fill in the spaces between the solvent particles. 2. type of solvent: Each solvent has different attractions to the type of solute. The stronger the attraction forces the more solute dissolved. Each solvent had different spaces between their particles. (not really true!!!) 3. temperature of solvent: as the temperature of the solvent increases, so does the spaces between the solvent particles, thus more room for more solute to dissolve. Thermal Pollution is when warmer water from factories is returned to lakes/streams. This causes a decrease in the dissolved gases (O2, CO2) meaning that aquatic organisms have less gas to survive. high Amount Dissolved g/mL low cold hot temperature ˚C