Chem Review- handout
advertisement

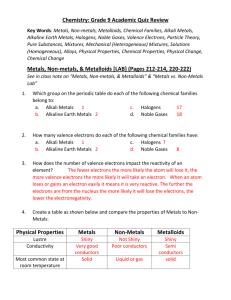
Chemistry: Grade 9 Academic Quiz Review Key Words: Metals, Non-metals, Metalloids, Malleable, Ductile, Conductivity, Chemical Families, Alkali Metals, Alkaline Earth Metals, Halogens, Noble Gases, Pure Substances, Mixtures, Mechanical (Heterogeneous) Mixtures, Solutions (Homogeneous),Quantitative Observations, Qualitative Observations, Alloys, Physical Properties, Chemical Properties, Physical Change, Chemical Change Metals, Non-metals, & Metalloids [LAB] (Pages 220-222, 224-227, 228229) See in class note on “Metals, Non-metals, & Metalloids” & “Metals vs. NonMetals Lab” 1. Which group on the periodic table do each of the following chemical families belong to: a. Alkali Metals c. Halogens b. Alkaline Earth Metals d. Noble Gases 2. Create a table as shown below and compare the properties of Metals to NonMetals: Physical Properties Metals Non-Metals Lustre Conductivity Most common state at room temperature Malleability or Brittleness Ductiliy Hardness a. What properties might you expect of metalloids? Explain why semi-conductors are useful for electronics. Mixtures and Pure Substances (Pages 190-191) See note Worksheet on “Pure Substance vs Mixture” 1. Which of the following sentences best explains why water evaporates at its boiling point: a. Water molecule become attracted to the air b. Water molecules move rapidly and spread further apart 2. 3. 4. 5. c. Water molecules become less attracted to one another when they are heated to high temperatures d. Water becomes too hot and needs to escape in order to cool off List 5 examples of pure substances and mixtures we have discussed in class or have done in a worksheet in class. Are alloys considered mixtures or pure substances? Can you find alloys on the periodic table? Name 3 examples of alloys (research if you need to). Is CaCO3 considered a pure substance or a mixture? Explain. Create a chart with 5 examples of a mechanical mixture (heterogeneous) and a solution (homogeneous). Properties of Matter (Pages 178-185) See note on “Properties of Matter” and Worksheet on “Chemical and Physical Properties of Matter” 1. Did Magnesium or Sulfur react with acid? 2. What is a conductivity apparatus made of? 3. Describe yourself using a chart of quantitative and qualitative descriptions. Textbook Practice Questions #1-25 (page 202-203) #2,3,5-15 (page 204-205)