SOLUTIONS Solutions are a homogeneous mixture of 2 or more
advertisement
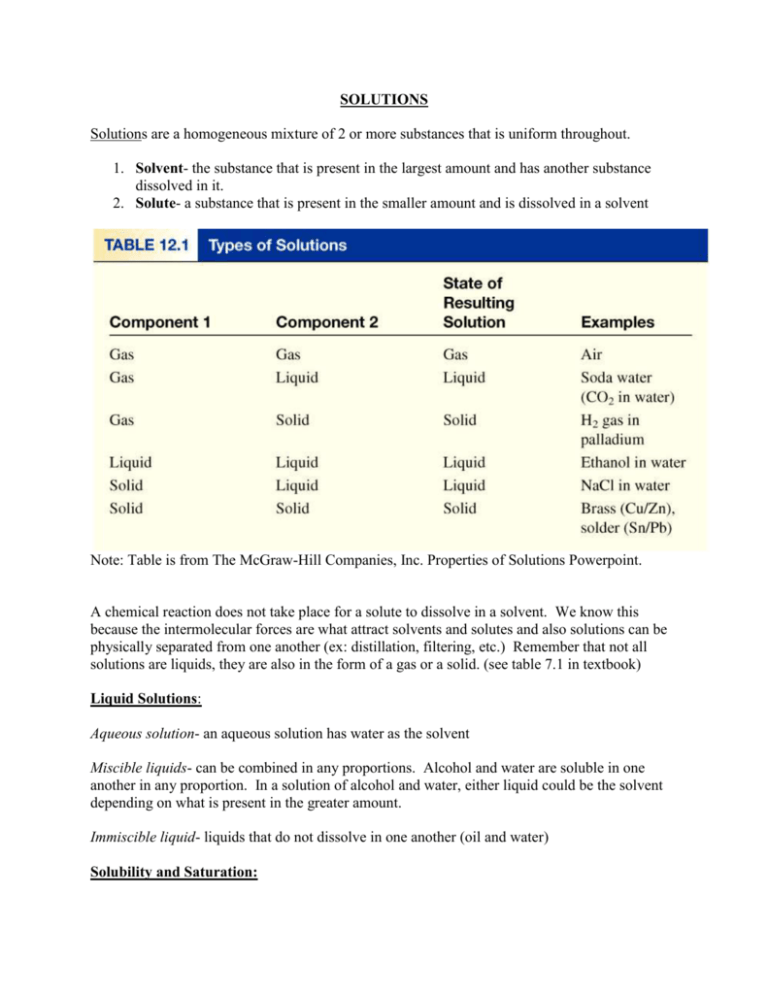
SOLUTIONS Solutions are a homogeneous mixture of 2 or more substances that is uniform throughout. 1. Solvent- the substance that is present in the largest amount and has another substance dissolved in it. 2. Solute- a substance that is present in the smaller amount and is dissolved in a solvent Note: Table is from The McGraw-Hill Companies, Inc. Properties of Solutions Powerpoint. A chemical reaction does not take place for a solute to dissolve in a solvent. We know this because the intermolecular forces are what attract solvents and solutes and also solutions can be physically separated from one another (ex: distillation, filtering, etc.) Remember that not all solutions are liquids, they are also in the form of a gas or a solid. (see table 7.1 in textbook) Liquid Solutions: Aqueous solution- an aqueous solution has water as the solvent Miscible liquids- can be combined in any proportions. Alcohol and water are soluble in one another in any proportion. In a solution of alcohol and water, either liquid could be the solvent depending on what is present in the greater amount. Immiscible liquid- liquids that do not dissolve in one another (oil and water) Solubility and Saturation: Solubility- the mass of solute that dissolves in a given quantity of solvent at a certain temperature. (Ex: 36g of NaCl can dissolve in 100mL of water at 20۫C Molar solubility- the amount in moles of dissolved solute in 1L solution Saturated solution- a solution in which no more solute will dissolve in a given amount of solvent at that temperature. Unsaturated solution- a solution in which more solute can still dissolve Super saturation solution - a solution containing more solute than is present in a saturated solution at a specific temperature. (A hot solution cooled very slowly so that excess solute will not precipitate.) Example: Sodium acetate crystals rapidly form when a seed crystal is added to a supersaturated solution of sodium acetate. Note: Image from The McGraw-Hill Companies, Inc. Properties of Solutions Powerpoint. What about solutes that “kind of” dissolve in solvents? When we say a solute is soluble, this generally means that the solubility is greater than 1g/100mL of solvent. Those that have a solubility less than 0.1g/100mL are considered insoluble. Slightly soluble substances are those that fall between 0.1-1g/100mL. Intermolecular forces: Dipole-Dipole interactions: the attraction between opposite charges on 2 different polar molecules. This is only 1% as strong as ionic or covalent bonds. Hydrogen bonding- dipole-dipole attractions when hydrogen is bonded to a very electronegative atom such as O, F, or N. This is stronger than dipole-dipole. Water is an example of a molecule that has hydrogen bonding. Ion-dipole- opposite charged ends of an ionic compound and polar molecule attract. Ion-dipole attraction must be greater than the bond within the ionic compound. (See dissociation/ion-dipole attraction animation) Dispersion (London) forces- a force between all molecules, however it is especially important for non-polar molecules and how they interact. Non-polar molecules can have a momentary distribution of charge to become polar and induces another polar molecule. The size of the molecule affects the strength of the force. Larger objects have a stronger dispersion force. Compound Lewis or Structural Diagram C2H2 NBr3 CH3F CH2O Shape Name Shape Diagram Polarity (P/NP) Min. 1 polar bond, and asymmetrical shape Types of Intermolecular Force (Dispersion, Dipole-dipole, Hydrogen bonding)










