The Dissolving Process handout
advertisement
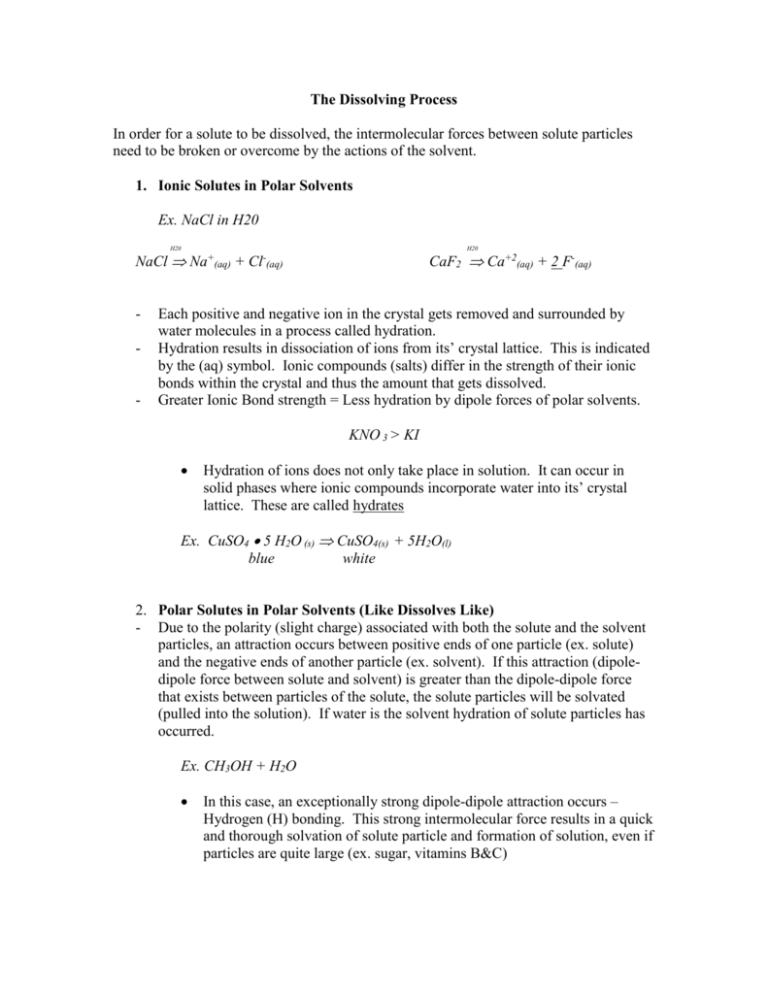
The Dissolving Process In order for a solute to be dissolved, the intermolecular forces between solute particles need to be broken or overcome by the actions of the solvent. 1. Ionic Solutes in Polar Solvents Ex. NaCl in H20 H20 NaCl - - H20 Na+(aq) + CaF2 Ca+2(aq) + 2 F-(aq) Cl-(aq) Each positive and negative ion in the crystal gets removed and surrounded by water molecules in a process called hydration. Hydration results in dissociation of ions from its’ crystal lattice. This is indicated by the (aq) symbol. Ionic compounds (salts) differ in the strength of their ionic bonds within the crystal and thus the amount that gets dissolved. Greater Ionic Bond strength = Less hydration by dipole forces of polar solvents. KNO 3 > KI Hydration of ions does not only take place in solution. It can occur in solid phases where ionic compounds incorporate water into its’ crystal lattice. These are called hydrates Ex. CuSO4 5 H2O (s) CuSO4(s) + 5H2O(l) blue white 2. Polar Solutes in Polar Solvents (Like Dissolves Like) - Due to the polarity (slight charge) associated with both the solute and the solvent particles, an attraction occurs between positive ends of one particle (ex. solute) and the negative ends of another particle (ex. solvent). If this attraction (dipoledipole force between solute and solvent) is greater than the dipole-dipole force that exists between particles of the solute, the solute particles will be solvated (pulled into the solution). If water is the solvent hydration of solute particles has occurred. Ex. CH3OH + H2O In this case, an exceptionally strong dipole-dipole attraction occurs – Hydrogen (H) bonding. This strong intermolecular force results in a quick and thorough solvation of solute particle and formation of solution, even if particles are quite large (ex. sugar, vitamins B&C) 3. Non polar solutes in Non polar Solvent (like dissolves like) Gasoline, oil, CCl4,etc. are non polar. So the dissolving process does not involve the interaction of charges and/or slight charges (polar substances). (No dipole-dipole forces; dipole-ion forces). Dissolving will occur as a result of mixing and natural random movement of solute/solvent particles because of the weak intermolecular (Van Der Waals) forces break readily. Ex. Oil & Gasoline (miscible liquids) Wax in Hexane Vitamins A,D,K in fatty tissues of the body The amount of mixing that occurs depends on the strength of the Van Der Waals forces among solute particles. These depend on: 1. Size of the particle (increase size = increased strength) 2. Shape of particle (geometry) Van der Waals' forces are observed in noble gases. Noble gases are very stable and tend not to interact, making them difficult to condense into liquid. However, the larger the atom of the noble gas, the easier it is to condense the gas. This is because a larger atom has a larger electron cloud, which more readily forms an ellipsoid, making the atom a temporary dipole. The electron cloud at any moment takes the shape of an ellipsoid around the nucleus, leaving a slight negative charge on one side of the major axis and a slight positive charge on the other. The dipole induces the same shift in neighboring atoms and spreads from one atom to the next. Unlike charges attract, and the induced dipoles are held together by dispersion force (or Van der Waals force). 4. Non polar solute in Polar Solvents – and Vice Versa - - In general the like dissolves like rule applies and the intermolecular forces between solute and solvent must be similar in order for the two to mix and form a solution. Usually the polar forces are too strong to be broken. Ex. Oil & Water (Immiscible Liquids) Ex. There are exception of the rule Styrofoam and acetone.





