SOLUTIONS and CONCENTRATIONS questions
advertisement

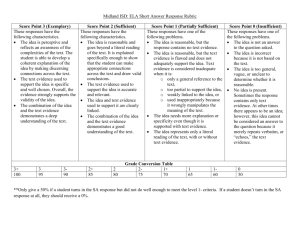
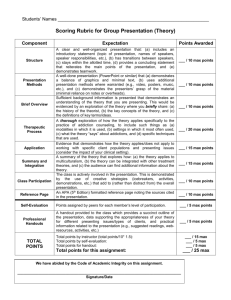
SOLUTIONS and CONCENTRATIONS ASSESSMENT (F) Name: ________________________________ 8____ date: __________________ OUTCOME #2: Investigate and describe the composition of fluids, and interpret the behavior of materials in solution. b• investigate the solubility of different materials, and describe their concentration Questions from page 28 of Science in Action textbook. (formative) 1. 2. What is the difference between a diluted solution and a concentrated solution? (use terms like solvent and solute) If a solution has a concentration of 75g per 100mL, what does this mean? 3. Calculate the concentrations in grams per 100mL: (show your work) Circle the new concentration. a. 2.1g/17mL b. 2.61g/18mL c. 2.78g/20mL d. Which solution (a, b, c) is more concentrated? 4. What is the difference between a saturated solution and an unsaturated solution? (use terms like solvent and solute) 5. What is the solute in a fruit punch drink? 6. What does soluble mean? Use the particle theory to explain. 7. Define insoluble and give two specific examples. Beginning Acceptable Proficient Mastery demonstrates limited academic achievement shows an incomplete understanding of the learning demonstrates basic academic achievement shows an adequate understanding and simplistic application of the learning demonstrates strong academic achievement shows a solid understanding and relevant application of the learning demonstrates exemplary academic achievement shows an in-depth understanding and insightful application of the learning in a variety of situations