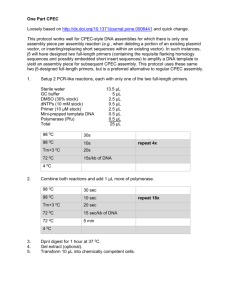
The presence of mtDNA of the kouprey in the DNA of the Thai Banteng
advertisement

Analysis of mitochondrial DNA of the D-loop region and haplotype mapping of Bos javanicus Drs. I.P.A. Dalm Studentnr: 3165159 January – April 2011 Supervisors: Dr. J.A. Lenstra Prof. W. Wajjwalku PhD student S. Dejchaisri Kasetsart University, Thailand Faculty of Veterinary Medicine, genetics, Nakom Pathom Research Internship C2001 1 Contents Abstract 3 Introduction 4 Materials and methods 7 Collection of the samples 7 DNA extraction from bone 7 DNA amplification using Polymerase Chain Reaction 8 Gel Electrophoresis 9 Sequencing 10 Data analysis 10 Results 11 Discussion 16 References 17 Attachments 19 2 Abstract The genetic relation between the different Bos javanicus (banteng) in Southeast Asia is in some ways uncertain. Bos javanicus is divided into three different groups: banteng from java, banteng from Borneo and banteng from the mainland of Southeast Asia. Some authors don’t agree with this division though. In this research I investigated if the Thai banteng contains mtDNA of the extinct kouprey, which was already shown in the Cambodian banteng21. In this research I also compared the genetic variation of seventeen different D-loop sequences of Bos javanicus, and I arranged them into different haplotypes. Because no haplotype classification has yet been published for Bos javanicus, I made my own denomination for the haplotypes. Eleven of the seventeen sequences were collected from dung in Huai Kha Khaeng national park in Thailand. The other six sequences were published data, and contained both mainland banteng and banteng from Java. After comparing the data, it was shown the sequences were divided into two well defined branches, one branch for the mainland banteng and one for the Javan banteng. Within the mainland banteng I found five different haplotypes (A, A2, A3, B, B2) and within the Javanese banteng one haplotype (C). So in total I found six different haplotypes between the seventeen sequences of Bos javanicus. Because the Thai banteng belongs to the same haplotype as the Cambodian banteng, it is certain the Thai banteng contains mtDNA of the kouprey as well. 3 Introduction The kouprey (bos sauveli) is a very rare or even extinct bovid species. It was found in the open forests of northern and eastern Cambodia and was discovered in 1937. The 2008 IUCN report lists the kouprey as Critically Endangered (possibly extinct), because the kouprey hasn’t been seen in the wild since 198322. Previous research shows the Cambodian banteng is related to the kouprey21. The banteng is a wild bovine species that lives in Southeast Asia. The wild banteng can currently be found on Java, possibly Bali, Kalimantan (the Indonesian part of Borneo), Sabah (Malaysian part of Borneo), Myanmar, Thailand, Lao, Vietnam and Cambodia. Around 1, 5 million banteng have been domesticated in several places in Southeast Asia and these are called Bali cattle1. Domestication of banteng probably took place around 3500 BC 2, 3. The wild banteng is registered on the red list of IUCN as endangered (figure 1)22. The exact number of the wild banteng population is thought unlikely to be more than 8000 animals and possibly less than 5000 animals. This is supported by the fact that there are no subpopulations bigger than 500 animals and there are six till eight subpopulation known of more than 50 animals. Java has got four or five of these subpopulations and Thailand two or three 4,5. These two subpopulations in Thailand are in Huai Kha Khaeng wildlife sanctuary, Khoa Ang Rue Nai wildlife sanctuary and perhaps Kaeng Krachan National park, because it is uncertain they contain more than 50 animals. The total number of wild banteng in Thailand is estimated to be around 300 till 500 animals. The total amount of wild banteng in Thailand probably declined by approximately 85% from 1980 to 2000 4. The banteng population in Huai Kha Khaeng wildlife sanctuary has increased over the past ten years tough and contains estimated at over 250 individual 6. The Khao Ang Rue Nai Wildlife Sanctuary, in eastern Thailand contains about 80 banteng. These two sanctuaries are the last ones in Thailand with a sizable banteng herd left. Other smaller populations of wild banteng in Thailand persist in Mae Ping National Park, Phu Luang Wildlife sanctuary, Thap Lan national park, Khwo Ang Ru Nai wildlife sanctuary, Khao Soi Dao wildlife sanctuary and Khoa Sok national park. However, these populations contain less than 50 animals tough. 4 Figure 1: the amount and spread of wild banteng in Southeast Asia due to IUCN in 09/30/2008 22 In Asia the tribe bovini contains three subtribes. The first one is the subtribe Bovina, which contains all species ranged into the two genera Bos and Bison. The second one is the subtribe Bubalina, which incorporates all species Bubalus and Syncerus. The last subtribe is called Pseudoryina, which only contains P. nghetinhensis. Bos javanicus includes in the subtribe Bovina with six other species 7. According to the classification of Wilson & Reeder (2005) 5 these six species are: Bos taurus, Bos. Frontalis (gaur), Bos grunniens (yak), Bos sauveli (kouprey), Bison bison (American bison) and wisent Bison bonasus (European bison). In the world there are three subspecies of Banteng recognized 8,9 1. B. javanicus javanicus. This banteng subspecie lives on Java and Bali 2. B. javanicus lowi on Borneo. This banteng subspecie lives on Borneo 3. B. javanicus birmanicus. This subspecie lives on the Asian mainland and is the subspecie my research is about. Some authors don’t agree about the separation of the banteng into three subspecies. They intend some small populations of wild banteng have been affected by interbreeding between domestic cattle or other wild cattle 1,10,11 Because the Javan banteng and the Asian mainland banteng differ phenotypically, the separating in subspecies are temporarily accepted. On the other hand, the phenotype of the B. javanicus lowi doesn’t differ that much from the Javan banteng and interbreeding between wild banteng and domestic cattle on Borneo is frequently reported 12,13 . This could be a reason for doubting the separation into subspecies. Bos javanicus has specific phenotypical characteristics and they also have sexual dimorphism. The females have a reddish-brown color with a dark dorsal stripe. Adult males have a blueblack or dark chestnut color, while younger males have a reddish-brown with a dark dorsal stripe, like the females. Bulls related to the Javan banteng are very dark brown to black. Regardless of sex, all Thai banteng have white stockings on all four legs, a white nose and a white patch on the rump. The banteng is 1.55 to 1.65 m and their build is similar to domestic cattle, but with a slender neck and smaller head. The horns of a female banteng are short and tightly curved and pointing inward while the horns of the males point upwards and are taller 14 . In this research I will use the medina D-loop sequences of the Thai banteng dung, which has earlier been executed by a Thai student at Kasetsart University. These eleven dung samples were collected from Huai Kha Khaeng national park. I will compare these sequences with DNA sequences of banteng from Genbank and arrange them into different haplotypes, because no different haplotypes have yet been published for Bos javanicus. I used mtDNA instead of nuclear DNA because mtDNA has a higher mutation rate and is therefore useful for studying the evolutionary relationships15. MtDNA is inherited maternally via the oocyte, instead of nuclear DNA which is inherited by both parents. Because mtDNA does not recombine, they change by chance mutation at each generation. These mutations arise when mistakes in the DNA sequence are copied. I used the D-loop part of the mtDNA in my research, because the D-loop gives the most information about the DNA diversity. This displacement loop or D-loop is a part of the mitochondrial genome which contains no structural genes and includes the two major transcriptional promoters and one of the origins of DNA replication 16. The D-loop consists of a short segment of three strands of DNA, the main two strands of DNA and an extra third strand of DNA which separates the two strands for a stretch, and thus is complementary to one of the main strands. 17 6 Materials and methods Collection of the samples In this research I used the bone of the Thai banteng to collect the DNA. I obtained the D-loop sequences of the mtDNA of the 20 bone samples. After comparing these sequences with other d-loop sequences of the mainland banteng, I found out my sequences were of the Javanese banteng. The 20 bone samples were collected by different rangers at Haui Kha Khaeng wildlife sanctuary in Thailand. This national park is located at the southern end of the Dawna Range, about 300 kilometers northwest of Bangkok. This World Heritage Site lies mainly in Uthai Thani Province, but it also extends into Tak Province. Because the bone samples were all of Javan banteng instead of mainland banteng, I used DNA sequences of different articles, Genbank and sequences of the university. DNA extraction from bone The bone which we use for our research is collected by rangers in the Huai Kha Khaeng national park. These were identified as originating from Thai banteng on the basis of the size of the bone of the head and the size and shape of the horns (figure 2). However, after sequencing I found out the bone was not from the Thai banteng but the Javan banteng Figure 2: picture of the skull of what was thought to be a banteng, but appeared to be a gaur The bone from the nostril area of the skull is kept in preserve media (EDTA) for two or three months, until the bone gets softer. The EDTA media is changed every week. To obtain DNA from the bone, the next steps were performed: The bone is washed with TE. A new tube contains lysis buffer, containing 900 μl 2x SSC, 100 μl 10 % SDS and 100 μl proteinase k. The buffer is added to the bone sample. The volume of the buffer is a little bit higher than the bone sample to get the best digestion. These samples are put in the heathbox for overnight heating at 56 ˚C. The next day the samples will be vortexed and centrifuged at 10.000 rpm for a period of 30 seconds. 1,300 μl L1 and 50 μl silica are put in a new tube, with 150 μl of the centrifuged supernatant. These tubes will shake for 3 hours at room temperature. Then they will be centrifuged at 10.000 rpm for 1 minute and the supernatant will be removed. After removing the supernatant, the tube will be washed 7 with 700 μl L2. To mix the pellet with the L2, vortex has to be used. The supernatant will be thrown away after centrifuging 11.00 rpm for 1 minute. These steps will be repeated. After this the same will be done, but instead of L2, 700 μl 70% ethanol will be used. This step must also be repeated. The pellet will be dried at 56 ˚C. 60 μl TE will be added and incubated for 5 minutes at 56 ˚C. The final step is centrifuging for 5 minutes with13.000 rpm. The DNA samples were stored at -20ºC DNA amplification using Polymerase Chain Reaction To amplify the obtained DNA, I used the technique polymerase chain reaction (PCR). With PCR we amplify a specific region of the DNA, in this case the D-loop of the mtDNA of the banteng. To accomplish PCR we need to have certain requirements. First of all we need a template, in this case DNA of the bone from a banteng. Second we need two primers. In this study we used two pairs of primers. The first pair is named primer bone D-loop forward for the forward strain, and D-loop reverse for the reverse strand. The two new made primers I also used are named DloopFW_bone for the forward strand and DloopRe_bone for the reverse strand. These primers were developed in the laboratory of Kasetsaert University. New primers: DloopFw_bone: 5’- GTA CAT AAC ATT AAT GTA ATA AAG ACA -3’ DloopRe_bone: 5’- GCA TGG TAA TTA AGC TCG TGA TC-‘3 Old primers: D-loopfw: 5’- CAC CCA TCA ACA CCC AAA gCT- 3’ D-loopre: 5’- CCT gAA gAA AgA ACC AgA TgC- 3’ In this study, the old primer was used for the forward replication (D-loopfw) and the new primer for the reverse replication (DloopRe_bone). To make sure all DNA of the D-loop was collected, I also used the new primer forward (DloopFw_bone) and the old primer reverse (Dloopre). These primers will amplify a 400 basepair fragment. The most important demands of a primer is the exclusiveness. Therefore you rather want a primer with more basepairs then a small one with similarities with the original piece. An ideal primer is for example between 18 and 22 basepairs, contains 50% GC nucleotides and consists of a random blend of nucleotides. Another important demand is the melting point of the primers. This needs to be not more than two degrees below the annealing temperature and certainly not above. The optimal melting point of a primer is between 50°C and 68 °C, and they need to be the same for both of the primers18. The last requirements to execute PCR are the ingredients for the mastermixture. For PCR of bone the mastermixture is as follows: Mastermixture PCR bone: Dw (water) 46 μl 8 10x buffer KCL2 + TAG buffer 10 μl MgCl2 8 μl 10 mM dNTP 2 μl Primer forward 1 μl Primer reward 1 μl Tag DNA polymerase 2 μl Procedure: The collected template DNA was put in Peltier Thermal Cycler-200 to accomplish PCR. In my research I used 40 cycles for PCR, which actually means 40 temperature changes. The different temperatures combined with the length of time, depend on the enzymes, NTPs and the melting temperature of the primers 18. The first step is the initialization or pre-denature step, which is only required for DNA polymerases that require heat activation. The second step is the denaturation step. This step causes melting of the DNA template by disturbing the hydrogen bonds. The following step is called the annealing step. In this step the temperature drops, so the primers can anneal to the single stranded DNA and start DNA synthesis. Next is the extension or elongation step. The temperature at this step is the optimum activity temperature for tag DNA polymerase, so DNA synthesis will be completed. To make sure any remaining single stranded DNA is fully extended, the extension/elongation step will be followed by a final elongation or lact extension step. The optimal conditions for PCR in my research are as follows: Conditions 40 cycles PCR bone: 1. 2. 3. 4. 5. 6. Pre-denature Denature Annealing Extension Lact extension Stop 95 C, 3 minutes 94 C, 30 seconds 50 C, 30 seconds 72 C, 90 seconds 75 C, 5 minutes 4 C, 0 minutes Gel electrophoresis To check if the PCR was performed correctly we use the technique gel electrophoresis. With this technique nucleic acid will be separated because of the electric field in the agarose matrix. Because of this electric field, negatively charged molecules move through the matrix, shorter ones faster than larger molecules. In this study the gel electrophoresis was performed with a 1,5% Agarose gel, a running time of 30 minutes, 100Volt and 100 mA. After the electrophoresis is completed, the gel will be put in a cup with ethidium bromide for 5 minutes, followed with 5 minutes of washing with water. Because of the ethidium bromide the DNA in the gel fluoresces under UV light. This is only possible when a band contains more than 20 ng 9 of DNA. When a visible band was produced, the negative control stayed negative, and no dimer primers were shown, the band was cut out of the gel and send for sequencing. Sequencing To obtain the DNA from the agar gel we used the silica protocol. This protocol can be found as attachment at the end of this thesis. After this protocol 2µL of supernatant of each sample was run again on agar gel, to check if the band was still visible. We didn’t do the sequencing ourselves, but this was performed by a routine laboratory in Bangkok. To make sure the sequencing could be done correctly, at least 10 μL PCR product has to be sent. Data analysis To study the phylogenetic relationships and protein function of the DNA sequences, I used the bioinformatical program Bioedit 4 to analyze the collected data. With this program the DNA sequences were aligned to identify homologous sequences and locate mutations. The evolutionary relationships between species are reproduced in a phylogenetic tree. To create this phylogenetic tree I used my obtained molecular data in the software program Mega 5. I also used mega 5 to blast the alignment from the bone sequences. With blast I could find the species my alignment from the bone sequences most connected, to prove the bone wasn’t from the Thai banteng. 10 Results: The primer pairs that were used in the article of Hassanin to collect the sequence of the Dloop of mitochondrial DNA are19: first pair: Forward: 5’-ACT-AAT-ACC-AAC-AGC-CGG-CAC-3’ Reverse: 5’-GAG-TAC-AAA-GTC-TGT-GTT-GAG-3’ Second pair: Forward: 5’-TAG-TTC-CAC-AAA-CGC-AAA-GAG-C-3’ Reverse: 5’-GTT-GCT-GGT-TTC-ACG-CGG-CAT-GG-3’ The primers used at Kasetsart University to obtain the sequences of the eleven faeces samples are: D-loopfw: 5’- CAC- CCA- TCA- ACA- CCC- AAA- gCT- 3’ D-loopre: 5’- CCT- gAA- gAA- AgA- ACC- AgA- TgC- 3’ I used the same primers from above for the bone samples, but I added other reverse and forward primers to obtain an overlap. The two extra primers were developed at the University and were called: Bone D-loop fw : 5’ -GTA-CAT-AAC-ATT-AAT-GTA-ATA-AAG-ACA 3’ Bone D-loop re: 5’- GCA-TGG-TAA-TTA-AGC-TCG-TGA-TC-‘3 I used primer D-loopfw with primer Bone D-loop reward and Bone D-loop forward with Dloopre (Figure 3). Figure 3. The location of the primers in the D-loop of the bone samples I compared the obtained sequences and extracted the overlap of around 200 basepairs. The obtained alignments of the overlap are shown in attachment 3. After comparing the alignments with other sequences from Genbank, I first found the sequences most matched with bos gaurus (96%). Just recently I found a better match with bos javanicus birmanicus (table 1), after a publication by Hassanin22. In earlier researches, other primers have been used to obtain D-loop sequences in bos javanicus birmanicus. That’s why I first couldn’t find a match with bos javanicus birmanicus. 11 Species Number Genbank Max ident Bos javanicus birmanicus JN632605 99% Bos gaurus DQ3770599 96% Bos javanicus javanicus JN632606 95% Bos frontalis DQ995680 94% Table 1. Comparison of bonesequences with sequences from Genbank I compared in total 17 DNA sequences of the mitochondrial D- loop of the different types of banteng. Eleven of these sequences (1,2,3,5,6,7,10,11,14,15,16) were collected from faeces found in Huai Kha Khaeng wildlife sanctuary. These DNA extractions were performed by a Thai student at Kasetsart university. The other 6 sequences I used were previously (Hassanin et al., 2010)19,23. The DNA from the article was extracted from fresh tissues (blood, muscle or hairs) or from bones from different types of banteng around the world (table 2). Species Bos javanicus birmanicus D-loop EF693806 Gender Male Species Cambodian banteng EF693808 Male Cambodian banteng EF693804 Male Cambodian banteng EF693807 Female Cambodian banteng EF693809 Male Javan banteng EF693805 Female Javan banteng Bos javanicus javanicus Location Teuk Chho Zoo, Cambodia Phnom Tamao Zoo, Cambodia Musée National d'Histoire Naturelle Phnom Tamao Zoo, Cambodia Zoo CERZA, Hermival-lesVaux Musée National d'Histoire Naturelle Bp 521 520 521 521 691 683 Table 2. Sequences from the D-loop of mtDNA from the article ‘resolving a zoological mystery: the kouprey is a real species’ by Hassanin et al. (2010) 12 I made an alignment of these DNA sequences of the D-loop in Bioedit so I could classify the used seventeen DNA sequences in the three different types of banteng species. After the alignment I could also range each group into different haplotypes. Alignment of the sequences showed that the 11 faeces samples were all from mainland banteng as expected. After amplifying and sequencing the D-loop region of the mtDNA, the single nucleotide polymorphism (SNPs) in this area could be identified. To identify these variations I aligned the seventeen sequences in the program bioedit (attachment 2). The sequence variations are caused by nucleotide substitutions, insertions or deletions20. Because of the SNPs the sequences can be arranged into different haplotypes (table 2). After investigating the obtained alignment I found in total 76 SNPs in the seventeen sequences with six different haplotypes (attachment 2 and table 3). I called the haplotypes A, A2, A3, B, B2 and C, because no haplotypes of Bos javanicus had yet been published. Haplotype A, A2, A3, B and B2 were all from mainland banteng. Haplotype C is of the Javan banteng. I made a phylogenetic tree of the seventeen different sequences (figure 3). It is shown that this tree contains two well defined branches, a branch for the mainland banteng and a branch for the Javan banteng. The branch of the mainland banteng contains the haplotypes A and B. The branch of the java banteng only contains haplotype C. So within the mainland banteng I found five different haplotypes (A, A2, A3, B,B2) and within the Javan banteng one haplotype (C). Because it is not clear if the faeces samples from the Huai Kha Khaeng national park were from different animals it is not possible to estimate haplotypes frequences in which the haplotypes occur.If you want to be certain the eleven faeces samples were all from different animals, it is necessary to analyze nuclear DNA. Haplotype Name Amount A 1,10,11, 14,15,16, EF693806 7 A2 EF693808 1 A3 EF693804, EF693807 2 B 3,5,6,7 4 B2 2 1 C EF693805, EF693809 2 17 Table 2: Different haplotype of Bos javanicus 13 Species Bos javanicus birmanicus Bos javanicus javanicus A A2 A3 B B2 C A A2 A3 B B2 C A A2 A3 B B2 C A A2 A3 B B2 C A A2 A3 B B2 C A A2 A3 164 G 184 A A A 201 C 204 A 222 T T T T G C 223 C 232 A 235 T 237 A 249 C T T C C C C G A 305 C 307 T 310 C 311 A 257 T 260 T 266 T C C C C 312 C 316 C 318 C T G A C C 267 G 283 G 286 A 290 A 291 A 295 C A A A A A A G G G G G C C C T T T T C A G C 338 A 340 A 341 C 342 A 343 C 345 C 346 C 347 C 349 C 350 C 351 A 354 G 355 C G T T T T T T A A T T A T T A T 513 G 516 T 525 C 536 T 547 T 565 T 570 T 573 G 582 A 588 A 601 C 603 T 606 C A A A C C A T T T C C C C G G G G G T 607 A 608 A 610 T 625 G 646 C 651 T G C C C C 662 C 664 T G G T T T T T 661 T 627 A C C A 628 C 629 T 630 C T T T G G T 670 G A A 679 A T C 686 T 687 T 14 703 C 632 T C T 653 T 654 G C A A C T 708 T 709 T 740 G - B B2 C C C C T T T C C C A A A G G C C C C A C C C C A A - Table 3: Sequence variation in 740 basepair fragments from 17 samples and rearranged into haplotypes 360674 BJ 16 D-loop re A 360666 BJ 10 D-loop re 360670 BJ 14 D-loop re EF693806 360672 BJ 15 D-loop re 360654 BJ 1 D-loop re Bos javanicus birmanicus 360668 BJ 11 D-loop re Ef693808 A2 EF693804 EF693807 360656 BJ 2 D-loop re A3 B2 360660 BJ 5 D-loop re 360662 BJ 6 D-loop re B 360658 BJ 3 D-loop re 360664 BJ 7 D-loop re Bos javanicus javanicus EF693805 C EF693809 0.1 Figure 4. phylogenetic tree of the seventeen DNA sequences of Bos javanicus and rearranged into the different haplotypes 15 Discussion In this research I investigated the hypothesis: the Thai banteng contains mtDNA of the extinct kouprey as found previously for the Cambodian banteng 7,21. Because the Cambodian banteng and the Thai banteng are both categorized in the subspecie B. javanicus Birmanicus, it was expected the Thai banteng would be related to the kouprey as well. The Javan banteng doesn’t contain DNA of the kouprey, because it’s a different subspecies. I used the sequences of the D-loop of mitochondrial DNA of banteng dung and other publications to arrange this species into different haplotypes. The bone samples I investigated for this research were of no use for this arrangement. This was because the part of the D-loop I obtained, was not investigated by other researchers. That’s why I first thought the used bone samples belonged to a different species (bos gaurus), because these sequences were most similar in Genbank (96%). Just recently a sequence of the same part of the D-loop of the mainland banteng was published by Hassanin22. This part shows a 99% similarity with my obtained sequences of the bone, which concludes the bonesamples does belong to the bos javanicus birmanicus. To make the bonesamples of any use in this research, published primers had to be used, so the same part of the D-loop could be obtained to make comparison possible. In this research I found six different haplotypes of Bos javanicus in seventeen different sequences. I arranged the six different haplotypes in A, A2, A3 (ten sequences), B, B2 (five sequences) and C (two sequences). The six haplotypes can be arranged into three main groups: A, B and C. Haplotype A and B are only found in mainland banteng. Haplotype C is only found in Javan banteng. Six sequences from the A haplotype were collected from the Haui Kha Khaeng national park in Thailand. The other four were collected from Cambodian banteng in Zoo’s and one from a museum. The five B haplotypes were all collected from Haui Kha Khaeng national park. The last two C haplotypes were both from Javan banteng. 16 References 1. Kusdiantoro Mohamad et al. (2009) On the Origin of Indonesian Cattle 2. Mohamad et al. (2007) Genetic Diversity and Conservation of South-East Asian Cattle: From Indian Zebu to Indonesian Banteng, and then to the Cambodian Kouprey 3. Felius, M (1995) Cattle breeds: an encyclopedia. 4. Hedges, S (2000) In press. Asian Wild Cattle and Buffaloes: Status Report and Conservation Action Plan. IUCN/SSC Asian Wild Cattle Specialist Group, Gland, Switzerland. 5. Pudyatmoko, S (2004) Does the banteng (Bos javanicus) have a future in Java? Challenges of the conservation of a large herbivore in a densely populated island. Knowledge Marketplace Reports of the 3rd IUCN World Conservation Congress: 6 pp.. Bangkok, Thailand. 6. Steinmetz, R (2004) Gaur (Bos gaurus) and banteng (B. javanicus) in the lowland forest mosaic of Xe Pian Protected Area, Lao P.D.R.: abundance, habitat use, and conservation. Mammalia 68: 141-157. 7. Hassanin, A, Ropiquet, A (2004) Molecular phylogeny of the tribe Bovini (Bovidae, Bovinae)and the taxonomic status of the Kouprey, Bos sauveli Urbain 1937 8. Lekagul and McNeely (1977) National Research Council 9. Byers, Hedges & Seal (1995) Asian Wild Cattle Conservation Assessment and Management Plan Workshop: Chonburi, Thailand: working document 10. Grzimek (1990) encyclopedia of mammals 11. Corbett, GB & Hill, JE (1992) The mammals of the Indomalay region: a systematic review. Natural History Museum Publications, Oxford University 12. Maarel, Van der (1932) Contribution to the Knowledge of the Fossil Mammalian Fauna of Java 13. Nijman et. Al (2003) Hybridization of banteng (Bos javanicus) and zebu (Bos indicus) revealed by mitochondrial DNA, satellite DNA, AFLP and microsatellites, Institute of Infectious Diseases and Immunology, Faculty of Veterinary Medicine, Utrecht University 14. Burnie D and Wilson DE (2005.), Animal: The Definitive Visual Guide to the World's Wildlife. DK Adult 15. Brown WM, George M Jr., Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76 (4): 1967–1971. 17 16. King, TC & Low, RL (May 5, 198) Mitochondrial DNA displacement loop structure depends on growth state in bovine cells; The Journal of Biological Chemistry, 262,6214-622 17. Kasamatsu, H et al. (1971) A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci USA' 68(9):2252-7). 18. Rychlik W, Spencer WJ, Rhoads RE (1990). Optimization of the annealing temperature for DNA amplification in vitro. Nucl Acids Res 18 (21): 6409–6412 19. Hassanin, A. Ropiquet, A (2010) resolving a zoological mystery: the kouprey is a real species 20. Lutz S, Weisser HJ, Heizmann J, Pollak S. Location and frequency of polymorphic positions in the mtDNA control region of individuals from Germany 21. What is the taxonomic status of the Cambodian banteng and does it have close genetic links with the kouprey? A. Hassanin, A. Ropiquet 22. Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. A. Hassanin Websites: 23. IUCN Redlist- International Union for the Conservation of Nature http://www.iucnredlist.org/details/2888/0 24. NCBI Genbank- National Centre for Biotechnology Information http://www.ncbi.nlm.nih.gov/ 18 Attachment 1: Silica protocol: Get DNA from the agar gel to amplify - 1,5 ml tube - Add 300 μl L1 buffer - Incubation: 10 minutes at 55 C. Vortex at 3 minutes and 1 minute. - Add 10 μl silica - 20 minutes on shaker - Centrifuge 10.000 rpm for 1 minute - wash supernatant - Add 500 μl L2 and vortex - Centrifuge 10.000 rpm for 1 minute - Wash supernatant - Add 500 μl L2 and vortex - Centrifuge 10.000 rpm for 1 minute - Wash supernatant - Add 500 μl 75 % ethanol - Centrifuge 10.000 rpm for 1 minute - Wash supernatant - Add 500 μl 75 % ethanol - Centrifuge 10.000 rpm for 1 minute - Wash supernatant - Dry for 20 minutes at 56 C till resident? Is dry - Add 18 μl TE - Vortex - Incubate for 2 minutes - Centrifuge 13.000 rpm for 3 minutes - Pipet supernatant (2 times 10 μl) in 1,5 ml tube - Centrifuge 13.000 rpm for 3 minutes - Pipet supernatant in (2 times 10 μl) 1,5 ml tube - Check band with 2 μl supernatant (template) and 3 μl gelstar, 1,5 % agar, 100 V 10 minutes 19 20 Attachment 2: 21 22 23 Attachment 3: 24 25