Matter Unit Notes
advertisement
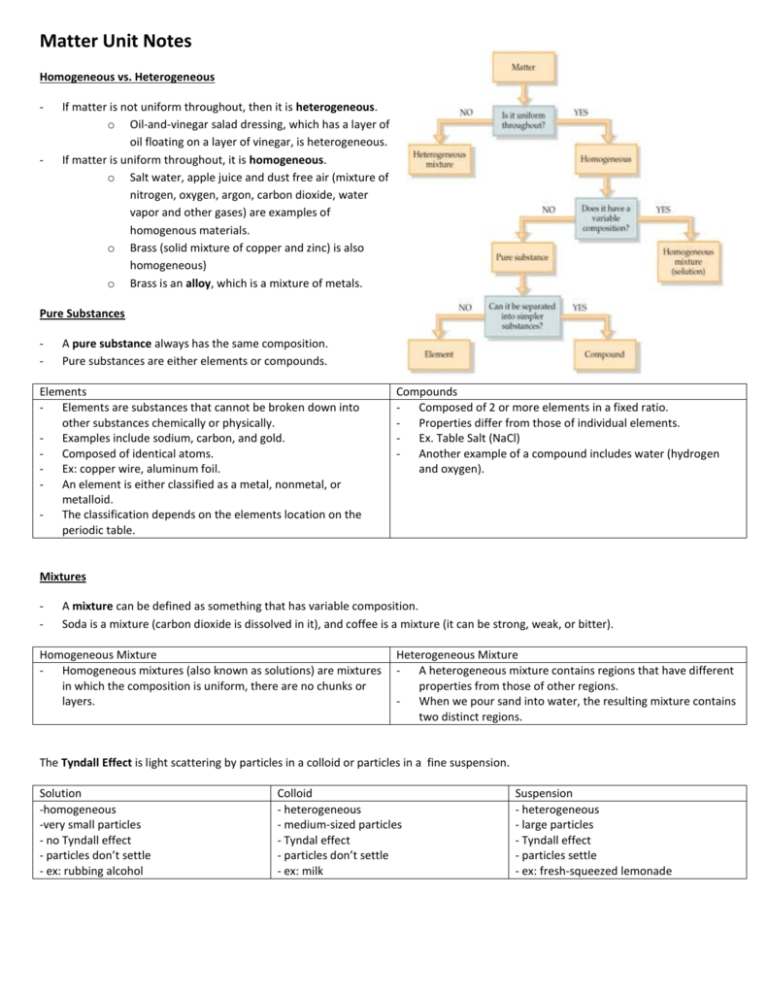
Matter Unit Notes Homogeneous vs. Heterogeneous - - If matter is not uniform throughout, then it is heterogeneous. o Oil-and-vinegar salad dressing, which has a layer of oil floating on a layer of vinegar, is heterogeneous. If matter is uniform throughout, it is homogeneous. o Salt water, apple juice and dust free air (mixture of nitrogen, oxygen, argon, carbon dioxide, water vapor and other gases) are examples of homogenous materials. o Brass (solid mixture of copper and zinc) is also homogeneous) o Brass is an alloy, which is a mixture of metals. Pure Substances - A pure substance always has the same composition. Pure substances are either elements or compounds. Elements Elements are substances that cannot be broken down into other substances chemically or physically. Examples include sodium, carbon, and gold. Composed of identical atoms. Ex: copper wire, aluminum foil. An element is either classified as a metal, nonmetal, or metalloid. The classification depends on the elements location on the periodic table. Compounds Composed of 2 or more elements in a fixed ratio. Properties differ from those of individual elements. Ex. Table Salt (NaCl) Another example of a compound includes water (hydrogen and oxygen). Mixtures - A mixture can be defined as something that has variable composition. Soda is a mixture (carbon dioxide is dissolved in it), and coffee is a mixture (it can be strong, weak, or bitter). Homogeneous Mixture Homogeneous mixtures (also known as solutions) are mixtures in which the composition is uniform, there are no chunks or layers. Heterogeneous Mixture A heterogeneous mixture contains regions that have different properties from those of other regions. When we pour sand into water, the resulting mixture contains two distinct regions. The Tyndall Effect is light scattering by particles in a colloid or particles in a fine suspension. Solution -homogeneous -very small particles - no Tyndall effect - particles don’t settle - ex: rubbing alcohol Colloid - heterogeneous - medium-sized particles - Tyndal effect - particles don’t settle - ex: milk Suspension - heterogeneous - large particles - Tyndall effect - particles settle - ex: fresh-squeezed lemonade States of Matter Solid Liquid Gas A solid is a form of matter A liquid is a form of matter A gas is a form of matter that has its own definite that flows, has constant that flows to conform to shape and volume. (definite volume, and takes the shape of its container A solid cannot flow the shape of the container. and fills the entire volume The particles can vibrate The particles in a liquid are of its container. but cannot move around. not rigidly held in place and Compared to solids and The particles or matter in a are less closely packed than liquids, the particles of solid are very tightly are the particles in a solid; gases are very far apart. packed. liquid particles are able to Because of the significant When a solid is heated the move past each other. amount of space between particles in the solid vibrate A liquid is not very particles, gases are easily more rapidly. compressible. compressed. The absorbed heat When liquids are heated As a gas (vapor) is increases the potential the potential energy of its compressed (the pressure energy of its particles particles increases even is increased), attractive (which is directly related to more, reducing the forces are increased. the space between attraction between As a result the gas can be particles). particles as well. converted into a liquid. This in turn reduces the attraction between particles so that they can flow in a liquid phase. The classification and properties of matter depend upon microscopic structure. - Plasma Plasma is composed of electrons and positive ions at temperatures greater than 5000o C. The sun and other stars are examples of plasma. In a plasma, the negatively charged electrons are freely streaming through the positively charged ions. Particle arrangement--------------------------------------------------------------------------------------------------------------------------------- Particle energy ------------------------------------------------------------------------------------------------------------------------------------- --- Particle to particle distance ------------------------------------------------------------------------------------------------------------------------ Vaporization vs. Evaporation - - Vaporization of an element or compound is a phase transition from the liquid phase to the gas phase. The two sorts of vaporization: evaporation and boiling. During evaporation, a liquid changes phase at its surface and becomes gas. During boiling a liquid changes phase at any place within the liquid, and gas bubbles form. Evaporation is a phase transition from the liquid phase to gas phase that occurs at temperatures below the boiling temperature at a given pressure. Most liquids are made up of molecules, and the levels of mutual (intermolecular) attraction amount molecules help explain why some liquids evaporate faster than others. As a result, liquids with strong intermolecular attractions evaporate more slowly than liquids with weak intermolecular attractions. For example, because water molecules have stronger mutual attractions than gasoline molecules, gasoline evaporates more quickly than water. The Solution Process - The formation of solutions is a physical change forming a homogeneous mixture. A solution is made up of a solute and a solvent. The solvent does the dissolving. The solute is the substance that is dissolved. Some things dissolve easier in one kind of substance as opposed to another. Sugar dissolves easily in water and oil does not. Water has a low solubility when it comes to oil. Since oil is not soluble in water, it will never truly dissolve. A solute will dissolve in a solvent if the solute-solvent forces of attraction are great enough to overcome the solute-solute and solvent-solvent forces of attraction. - A solute will not dissolve if the solute-solvent forces of attraction are weaker than individual solute and solvent intermolecular attractions. “Like dissolves like”: the expression means that dissolving occurs when similarities exist between the solvent and the solute. o Water is polar, while C6H14 and CCl4 are nonpolar. As a result, neither substances will dissolve in water. Concentration of Solutions - Solubility- the maximum amount of substance that will dissolve at that temperature. o If the amount of solute dissolved is less than the maximum that could be dissolved, the solution is called an unsaturated solution. o A solution which holds the maximum amount of solute per amount of the solution under the given conditions is called a saturated solution. o A supersaturated solution contains more solute than the usual maximum amount and is unstable. They cannot permanently hold the excess solute in solution and may release it suddenly. Solubility and Temperature - Increasing the temperature of a solvent speeds up the particle movement. This causes more solvent particles to bump into the solute, resulting in solute particles breaking loose and dissolving faster. Solubility Curve - A graph of the solubility of a compound (grams/100 grams of water on the Y-axis) at various temperatures (Celsius on X-axis). Each compound has a different curve. Solubility is dependent on temperature. Solids are more soluble at…higher temperatures. Gases are more soluble at …lower temperatures and higher pressures. Density - Density is the amount of matter (mass) contained in a unit of volume. (Styrofoam has a low density or small mass per unit of volume). Density = mass/volume o Solving density problems Cover the variable you are solving for. If you cover the top variable, multiple the bottom ones. To solve for mass, multiply volume by density. If you cover a bottom variable, take the top variable and divide by the bottom. To solve for volume, take the mass and divide by the density. Physical Properties - Physical properties are characteristics that a sample of matter exhibits without any change in its identity. This property can be observed and measured without changing the substance. Examples of the physical properties of a chunk of matter include its: solubility, boiling point, melting point, density, color, magnetic, electrical conductivity, and physical state (solid, liquid, or gas). Chemical Properties - - Chemical Properties are properties of matter that describes a substance’s ability to participate in chemical reactions. A chemical property describes how a substance changes into a new substance, either by combining with other elements or breaking apart into new substances. Examples: o Reactivity: the ability of a substance to combine chemically with another substance. o Flammability: the ability of a substance to react in the presence of oxygen and burn when exposed to a flame. o In general, the ability of a substance to react…to form… You can observe chemical properties only in situations in which the identity of the substance changes. Properties of Metals and Nonmetals - - - Metals Metals are elements that have luster, conduct heat and electricity, and usually bend without breaking. Metals are also ductile (can be drawn out into a wire). All metals except mercury (Hg) are solids at room temperature; in fact, most have extremely high melting points and high boiling points. A metal’s reactivity is its ability to react with another substance. Metals in the first and second column of the periodic table are more reactive than other metals. - - - - Nonmetals Although the majority of elements in the periodic table are metals, many nonmetals are abundant in nature. Most nonmetals don’t conduct electricity, are much poorer conductors of heat than metals, and are brittle when solid. Many are gases at room temperature; those that are solids lack the luster of metals. Their melting points tend to be lower than those of metals. Fluorine is the most reactive nonmetal. - - - - Metalloids Metalloids have some chemical and physical properties of metals and other properties of nonmetals. In the periodic table, the metalloids lie along the border between metals and nonmetals. Some metalloids such as silicon, germanium (Ge), and arsenic (As)are semiconductors. A semiconductor is an element that does not conduct electricity as well as a metal, but does conduct slightly better than a nonmetal.