Cornell Notes Topic/Objective: CHAPTER 3 Section 3 Elements
advertisement
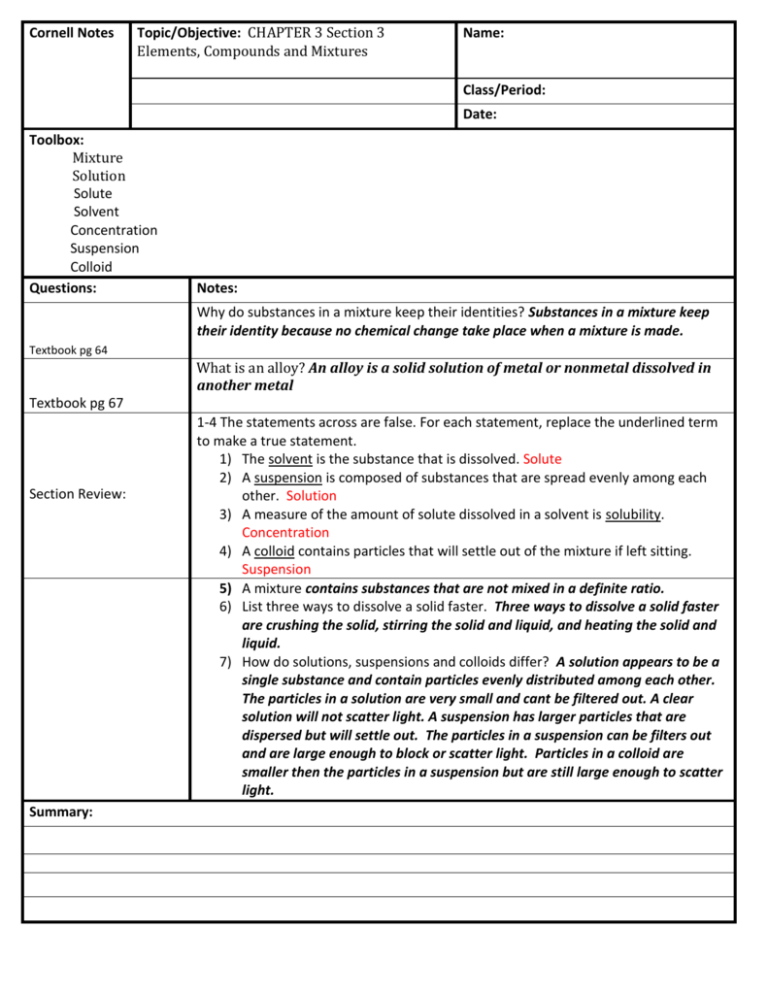
Cornell Notes Topic/Objective: CHAPTER 3 Section 3 Elements, Compounds and Mixtures Name: Class/Period: Date: Toolbox: Mixture Solution Solute Solvent Concentration Suspension Colloid Questions: Notes: Why do substances in a mixture keep their identities? Substances in a mixture keep their identity because no chemical change take place when a mixture is made. Textbook pg 64 What is an alloy? An alloy is a solid solution of metal or nonmetal dissolved in another metal Textbook pg 67 Section Review: Summary: 1-4 The statements across are false. For each statement, replace the underlined term to make a true statement. 1) The solvent is the substance that is dissolved. Solute 2) A suspension is composed of substances that are spread evenly among each other. Solution 3) A measure of the amount of solute dissolved in a solvent is solubility. Concentration 4) A colloid contains particles that will settle out of the mixture if left sitting. Suspension 5) A mixture contains substances that are not mixed in a definite ratio. 6) List three ways to dissolve a solid faster. Three ways to dissolve a solid faster are crushing the solid, stirring the solid and liquid, and heating the solid and liquid. 7) How do solutions, suspensions and colloids differ? A solution appears to be a single substance and contain particles evenly distributed among each other. The particles in a solution are very small and cant be filtered out. A clear solution will not scatter light. A suspension has larger particles that are dispersed but will settle out. The particles in a suspension can be filters out and are large enough to block or scatter light. Particles in a colloid are smaller then the particles in a suspension but are still large enough to scatter light. Questions: Notes: 8) Suggest a procedure to separate iron filings from sawdust. Explain why this procedure works. I would use a magnet to separate the iron from the sawdust. The magnet will attract the iron but will not attract the sawdust. 9) Identify the solute and solvent in a solution made of 15mL of oxygen and 5 mL of helium. The solute is helium and the solvent is oxygen. 10) At what temperature is 120g of sodium nitrate soluble in 100mL of water? About 60 degrees Celsius. 11) At 60 degrees Celsius, how much more sodium chlorate than sodium chloride will dissolve in 100mL of water? About 120g more of sodium chlorate will dissolve. Summary: