Mid-Term Review Sheet
advertisement

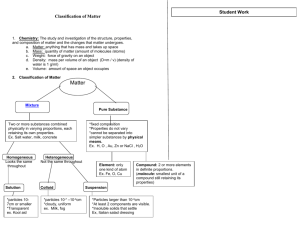
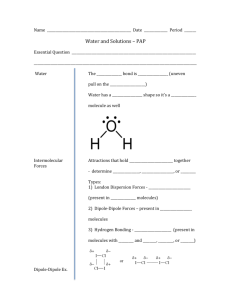
ChemComm Mid-Term Exam Review Sheet I. Water A. Matter, Measurement, and the Metric System 1. properties a. physical vs. chemical b. special properties of water 2. change a. physical vs. chemical b. dissolving c. neutralization 3. states of matter a. movement of particles b. properties c. effect of temperature 4. appropriate units of measurement 5. laboratory equipment B. Water Use and Supply 1. individual vs. regional use 2. hydrologic cycle 3. locations on earth (ice caps, etc.) C. The Periodic Table 1. symbol 2. atomic number 3. atomic mass / weight 4. metals, non-metals, metalloids 5. diatomic elements 6. Metals, non-metals, and metalloids a. physical properties b. chemical properties 7. groups / families 8. periods D. Compounds 1. ionic vs. covalent 2. formula a. symbol b. subscript 3. name E. Mixtures 1. solution - homogeneous a. solvent and solute b. factors affecting solubility c. solubility curves d. levels of saturation 2. heterogeneous a. colloid 1. Tyndall effect 2. small particles that cannot be filtered b. suspension 1. Tyndall effect 2. settling out 3. larger particles that can be filtered F. Our Water 1. dissolved oxygen a. aeration b. oxygen consumption c. oxygen production d. effects of dissolved oxygen levels on life 2. heavy metal ions a. identify b. individual effects c. EPA limits G. Purification and Treatment of Water 1. differences between 2. types of filtration 3. distillation 4. municipal water treatment 5. municipal water purification 6. natural purification H. The Riverwood Fish Kill II. Materials 1. Moles a. 6.02x1023 particles = 1 mole b. molar mass c. unit analysis 2. Law of Conservation of Matter a. Balancing equations b. Stoichiometry 3. Resources a. Renewable vs. non-renewable b. natural vs. man-made or engineered 4. Resources – where, why and how? a. Where are resources found? 1. atmosphere 2. hydrosphere 3. lithosphere Types of problems: 1. 2. 3. 4. 5. 6. 7. 8. metric conversions percentage solubility (how much solute dissolves in 300 g of water…) writing balanced equations (regular, redox, nuclear) molar mass stoichiometry percent composition finding oxidation states Don’t forget to review the following labs: 1. 2. 3. 4. 5. 6. 7. 8. 9. Density and what floats on what Tyndall effect Water softening Foul water purification Metal, non-metal, metalloid Signs of chemical change Activity series Penny (Gold, silver, and bronze) lab Oxidation States – Mn and N