ChemistryName: Ms. Boon Period: ______ Date: ______ Unit 1
advertisement

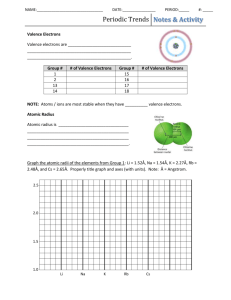
Chemistry Ms. Boon Name: _______________________ Period: _______ Date: __________ Unit 1, Lesson 6: Investigation – Periodic Trends Straw Model INTRODUCTION A trend is a predictable change in a certain direction. Understanding periodic trends allows us to make predictions about the chemical behavior of the elements. In this investigation, your team will create a 3D model of one periodic trend. PRE-LAB NOTES The three trends we are learning about today involve ionization energy, atomic radius, and electronegativity. 1. What is ionization energy? 2. What is atomic radius? 3. What is electronegativity? INVESTIGATION PROCEDURE SAFETY PRECAUTION Do NOT touch the metal tip of the hot glue gun or the hot glue at any time. There is a very limited supply of glue, scissors, and straws so please use these materials carefully. MATERIALS Straws Scissors Periodic Table Hot Glue Gun Periodic Trend Data Chart Ruler PROCEDURE 1. Your group will receive a periodic trend data chart. Follow the directions on the chart. Your group is responsible for building one model of one periodic trend. If you would like, arrange the straw colors by metals, nonmetals and metalloids or by groups or by periods. 2. After completing your model, identify the direction of the trend and add this information to your model. Your model must have two trend statements. The trend statements should be structured as follows: (Choose one: Electronegativity/Ionization energy/atomic radius) (choose one: increases/decreases) as we move down a periodic table group. (Choose one: Electronegativity/Ionization energy/atomic radius) (choose one: increases/decreases) as we move across a periodic table period from left to right. Chemistry Ms. Boon Name: _______________________ Period: _______ Date: __________ POST-LABORATORY QUESTIONS Every group member must complete the post-lab questions. You may work together. You will be asked to share your periodic table model and trend with the class. 1. What trend did your group model? 2. Use your trend data to make a graph of your trend for the Period 2 elements. Draw the graph in the space below. (X-axis: atomic # or element symbol; Y-axis: trend data) 3. Use your trend data to make a graph of your trend for the Group 1 elements. Draw the graph in the space below. (X-axis: atomic # or element symbol; Y-axis: trend data) 4. Find the textbook section on your group’s trend (ionization energy p. 133-134, atomic radius p. 135-136, electronegativity p. 136-138). Summarize the reasoning behind the trend your group observed. Chemistry Ms. Boon Name: _______________________ Period: _______ Date: __________ Notes on periodic trends: Take notes on the trends other groups observed here. What is the trend? Why does this happen? What is the trend? Why does this happen? Practice Questions 3. Put the following in order of increasing atomic radiusa. Mg, P, S, Cl b. N, Se, Po, Te c. Cs, K, As, In d. Na, Pb, Se, Ne 4. Put the following in order of increasing electronegativitya. Mg, P, S, Cl b. N, Se, Po, Te c. Cs, K, As, In d. Na, Pb, Se, Ne 5. Put the following in order of increasing ionization energya. Mg, P, S, Cl b. N, Se, Po, Te c. Cs, K, As, In d. Na, Pb, Se, Ne