Savor-TIMI-53-Saxagliptin-CV
advertisement
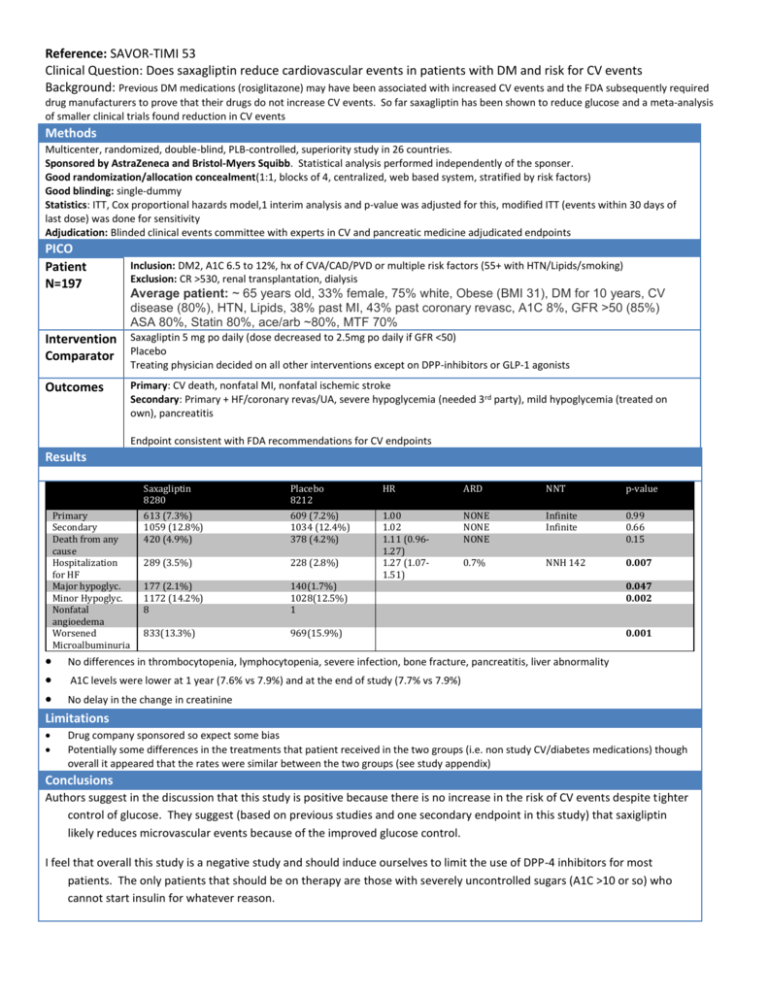
Reference: SAVOR-TIMI 53 Clinical Question: Does saxagliptin reduce cardiovascular events in patients with DM and risk for CV events Background: Previous DM medications (rosiglitazone) may have been associated with increased CV events and the FDA subsequently required drug manufacturers to prove that their drugs do not increase CV events. So far saxagliptin has been shown to reduce glucose and a meta-analysis of smaller clinical trials found reduction in CV events Methods Multicenter, randomized, double-blind, PLB-controlled, superiority study in 26 countries. Sponsored by AstraZeneca and Bristol-Myers Squibb. Statistical analysis performed independently of the sponser. Good randomization/allocation concealment(1:1, blocks of 4, centralized, web based system, stratified by risk factors) Good blinding: single-dummy Statistics: ITT, Cox proportional hazards model,1 interim analysis and p-value was adjusted for this, modified ITT (events within 30 days of last dose) was done for sensitivity Adjudication: Blinded clinical events committee with experts in CV and pancreatic medicine adjudicated endpoints PICO Patient N=197 Inclusion: DM2, A1C 6.5 to 12%, hx of CVA/CAD/PVD or multiple risk factors (55+ with HTN/Lipids/smoking) Exclusion: CR >530, renal transplantation, dialysis Average patient: ~ 65 years old, 33% female, 75% white, Obese (BMI 31), DM for 10 years, CV disease (80%), HTN, Lipids, 38% past MI, 43% past coronary revasc, A1C 8%, GFR >50 (85%) ASA 80%, Statin 80%, ace/arb ~80%, MTF 70% Intervention Saxagliptin 5 mg po daily (dose decreased to 2.5mg po daily if GFR <50) Comparator Placebo Treating physician decided on all other interventions except on DPP-inhibitors or GLP-1 agonists Outcomes Primary: CV death, nonfatal MI, nonfatal ischemic stroke Secondary: Primary + HF/coronary revas/UA, severe hypoglycemia (needed 3rd party), mild hypoglycemia (treated on own), pancreatitis Endpoint consistent with FDA recommendations for CV endpoints Results Primary Secondary Death from any cause Hospitalization for HF Major hypoglyc. Minor Hypoglyc. Nonfatal angioedema Worsened Microalbuminuria Saxagliptin 8280 Placebo 8212 HR ARD NNT p-value 613 (7.3%) 1059 (12.8%) 420 (4.9%) 609 (7.2%) 1034 (12.4%) 378 (4.2%) NONE NONE NONE Infinite Infinite 0.99 0.66 0.15 289 (3.5%) 228 (2.8%) 0.7% NNH 142 0.007 177 (2.1%) 1172 (14.2%) 8 140(1.7%) 1028(12.5%) 1 1.00 1.02 1.11 (0.961.27) 1.27 (1.071.51) 833(13.3%) 969(15.9%) 0.047 0.002 0.001 No differences in thrombocytopenia, lymphocytopenia, severe infection, bone fracture, pancreatitis, liver abnormality A1C levels were lower at 1 year (7.6% vs 7.9%) and at the end of study (7.7% vs 7.9%) No delay in the change in creatinine Limitations Drug company sponsored so expect some bias Potentially some differences in the treatments that patient received in the two groups (i.e. non study CV/diabetes medications) though overall it appeared that the rates were similar between the two groups (see study appendix) Conclusions Authors suggest in the discussion that this study is positive because there is no increase in the risk of CV events despite tighter control of glucose. They suggest (based on previous studies and one secondary endpoint in this study) that saxigliptin likely reduces microvascular events because of the improved glucose control. I feel that overall this study is a negative study and should induce ourselves to limit the use of DPP-4 inhibitors for most patients. The only patients that should be on therapy are those with severely uncontrolled sugars (A1C >10 or so) who cannot start insulin for whatever reason.











