CHF-Saxagliptin/DPP
advertisement

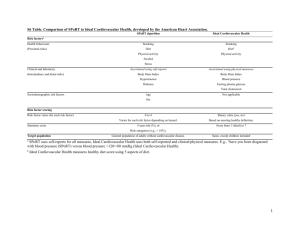
Suite 305 233 E. Lancaster Ave. Ardmore, PA 19003 , Affiliate, MLHS Office: 610-642-6800 Fax: 610-642-6850 E-mail: stschwar@gmail.com www.schwartzdiabetesdoc.com Diabetes, Cardio-Metabolic Newsletter CHF/SAXAGLIPTIN/ DPP-4 INHIBITORS 3/31/2014 The glycemia-lowering drug saxagliptin led to a “real” excess of hospitalizations for heart failure in a 16,000-patient trial designed to examine the drug’s cardiovascular safety, but given saxagliptin’s overall safety in the study the signal of heart failure exacerbation should not rule out the drug’s use for most patients, even those with preexisting heart failure. NOTE, RISK OF CHF WAS NOT INCREASED VS. PLACEBO.This follow-up analysis of data collected in the SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction) trial occurred because prior reports on the study’s outcomes had shown a small but statistically significant excess incidence of hospitalization for heart failure among the 8,280 patients treated with saxagliptin, compared with the 8,212 patients randomized to placebo. Researchers ran the SAVOR-TIMI 53 trial in patients with type 2 diabetes and either established cardiovascular disease or multiple risk factors to explicitly test the cardiovascular safety of saxagliptin (Onglyza), a selective dipeptidyl peptidase 4 (DPP-4) inhibitor, following guidance from the Food and Drug Administration in 2009 (N. Engl. J. Med. 2013;369:1317-26). The primary outcome from the study showed no statistically significant difference in the combined rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke between the saxagliptin and placebo arms during a median 2.1 years of follow-up. The results also showed saxagliptin met the FDA’s recommended safety criterion for cardiovascular safety in a drug meant to treat type 2 diabetes.More detailed analyses showed a 0.7% absolute increased risk for hospitalization for heart failure in the saxagliptin-treated patients, compared with the placebo group (a 27% increase in relative risk), and that the largest number of increased hospitalizations occurred among the quartile of patients enrolled in the trial with baseline blood levels of NT–pro-brain natriuretic peptide of at least 333 pg/mL. Further analysis reported for the first time by Dr. Scirica in his poster showed that the greatest excess rate of hospitalization for heart failure occurred during the first 6 months of treatment. After the first 6 months, hospitalization rates for this reason were virtually identical in the two treatment arms of the study, said Dr. Scirica, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School in Boston. His analysis also compared the impact of saxagliptin on hospitalization for heart failure with the same effect of alogliptin, another DPP-4 inhibitor, in results from the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin vs. Standard of Care) trial (N. Engl. J. Med. 2013;369:1327-35). In EXAMINE the 2,701 patients who received alogliptin (Nesina) had an absolute 0.6% higher rate of hospitalizations for heart failure (an 18% relative increase) that was not statistically significant. EXAMINE also showed no significantly different rate between alogliptin and placebo treatment for the study’s primary, combined cardiovascular endpoint during a median 1.5 years of treatment in patients with type 2 diabetes and acute coronary syndrome.“One could look at the alogliptin results and say there was no signal [for increased hospitalization for heart failure], or that there was a signal, although not statistically significant. The TECOS (Sitagliptin Cardiovascular Outcome Study) study using sitagliptin (Januvia), a third DPP-4 inhibitor, is slated to finish at the end of 2014. “Whether this is a drug or class effect still very much has yet to be seen,” said Dr. Scirica. The mechanism also remains elusive for the time being.“The most important message is that future trials [of diabetes drugs] need to prospectively look at heart failure rates, adjudicate heart failure events, and really look for any heart failure effect,” Dr. Bhatt said. THERE IS A LOGIC FOR DPP-4 INHIBITORS TO DECREASE CV OUTCOMES , PREVENTING DESTRUCTION OF PROTEINS THAT DECREASE ENDOTHELIAL DYSFUNCTION/ INFLAMMATION. META-ANALYSIS OF STUDIES USED TO EVALUATE DRUG EFFICACY SHOWED DECREASE CVEVENTS. MOREOVER, IN CATH STUDIES, 1 PILL OF SITAGLIPTIN DECREASES AREA OF MUSCLE AT RISK, INCREASES EF. SO, PERSONALLY I’M NOT WORRIED ABOUT SAXAGLIPTIN OR OTHER DPP-4 INHIBITORS