Pediatric A1C Levels– Where We Are
advertisement

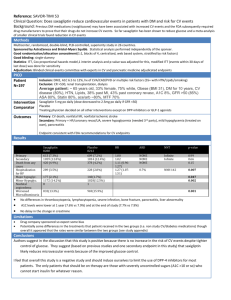
Why do we Need an Artificial Pancreas? Why do we Need an Artificial Pancreas? QuickTime™ and a decompressor are needed to see this picture. Georgeanna J Klingensmith, MD Barbara Davis Center Keystone 2010 Georgeanna J Klingensmith, MD Barbara Davis Center Keystone 2010 Why do we need an artificial pancreas? • Current glycemic targets • Current glycemic outcomes fall short • What factors contribute to achieving target glycemia • Can current technology allow youth to reach target glycemia? ADA Glycemic Guidelines Targets Age < 6yrs 6-12 yrs 13-18 yrs adult A1c % ** 7.5-8.5 <8 <7.5 <7 ** Goals should be individualized, a lower A1C goal is reasonable based on benefit:risk assessment and if it can be achieved without excessive hypoglycemia Silverstein, et al. Care of Children with type 1 DM. Diabetes Care, 28:186212, 2005 ADA Standards of Care. Diabetes Care, Suppl 1, 2010 ISPAD Glycemic Guidelines for all children and adolescents A1C = <7.5% for all children All targets should be individualized Higher targets are recommended in those with severe hypoglycemia and/or hypoglycemia unawareness Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and Monitoring of Glycemic Control. ISPAD clinical consensus guidelines 2009. Ped Diab 2009 Risk of Progression of Complications: Related to Glycemia, the DCCT Study Severe hypoglycemia 15 Diabetic retinopathy Nephropathy Neuropathy Microalbuminuria 120 Rate of 100 Severe Hypo. 80 (per 100 60 patient -years) 40 13 11 Relative Risk 9 7 5 3 20 0 GJ Klingensmith 1 6 7 8 9 10 HbA1c, % 11 12 DCCT Research Group. N Engl J Med. 1993;329:977- The DCCT: A1C can be modified and Technology Lowers A1C 1 DCCT Intervention DCCT 2 3 4 5 6 EDIC Observation S t u d y Y e a r 7 8 9 Where are we in the ‘real world’ of pediatric diabetes? The Hvidore Study Group on Childhood Diabetes A1C for participating centers 9.5 9.0 Mean +/- SE % A1c 8.5 Tosoh method 8.0 7.5 Mean A1C 8.2 + 1.4%, 7.0 Diabetes Care, 30:2245-50, 9/2007 SEARCH for Diabetes A1c Results 6 clinical centers in US Mean duration 5 years Mean 8.33% N=2999 8.24% N=369 “Good”: age specific ADA A1c target “Poor”: A1c ≥ 9.5%. “Intermediate”: 1c between “good” and “poor” Petitti D, et al, J Peds, Nov 2009 Factors associated with A1C in Multifactorial Analysis • • • • • Age, DM duration Insurance status Household income Parental education Race/ ethnicity < 0.001 < 0.001 < 0.001 < 0.001 < 0.001 When the HbA1c is adjusted for all of these factors, mean A1C for T1D = 8.0% Petitti D, et al, J Peds, Nov 2009 Insulin Regimen in SEARCH Type 1, N=2743, duration >1 year Insulin Regimen Pump A1C Race Asian/Pacific Isl. Black Hispanic Native American White 8.0 (1.1) MDI: Basal/Bolus 8.5 (1.6) MDI: Modified B/B 8.9 (1.6) MDI: 8.6 (1.6) 1-2 injections 8.6 (1.7) <.0001 Difference remains significant when controlling for socio-economic factors Row % 6.1 5.3 12.3 0.0 26.3 38.9 13.8 17.2 27.8 26.4 6.1 5.3 8.0 11.1 11.2 20.4 29.3 28.6 31.3 46.3 <.0001 31.3 22.2 11.7 38.9 24.4 Journal of Pediatrics Aug 2009 Factors associated with HbA1c in Multifactorial Analysis • • • • • • • Age, DM duration < 0.001 Insurance status < 0.001 Household income < 0.001 Parental education < 0.001 Race/ ethnicity < 0.001 Frequency of BG testing < 0.001 Type of insulin/ insulin delivery <0.001 Improvement in HbA1c levels %A1c The Joslin Clinic 9.2 9.1 9.0 8.9 8.8 8.7 8.6 8.5 8.4 8.3 8.2 9.0 8.7 8.4 Baseline 8.7 Cohort 2 used more intensive management with more frequent SBGM and Year 2 more on MDI and CSII Cohort 1 (1997-2000) Cohort 2 (2002-2005) A1c significantly lower for Cohort 2 compared to Cohort 1 at baseline (p=0.03) and two-year follow-up (p=0.04) J Pediatr. 2007 Mar;150(3):279-85 Does the latest technology help adolescents? • JDRF sensor study • Randomized controlled trial of sensor use vs standard care • 3 age groups – >25 – 15-25 – 8-14 N Engl J Med. 2008. 359(14):1464-76 86% 30% Only the >25 group had an improvement in A1C 50% Diabetes Care, 11/2009, 32 (11), p. 1952 Age Differences in Sensor use at 6 months % sensor >6d/wk >25 yrs 64% 15-25 yrs 19%* 8-14 yr 25% *21% of 15-24 year olds were not wearing the sensor at 6 months Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the JDRF-CGM trial Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Diabetes Care. 2010 Jan;33(1):17-22 Who successfully uses CGM • Those >25 years (p<0.001) • Those who test more frequently prior to initiation of CGM (p<0.001) • Those who wore the device >6 days a week in the initial month (p<0.001) • Those who achieved a greater percent of glucose values of 70-180mg/dl in month 1 (p<0.002) Diabetes Care 2009;32: 1947-1953 Do we need an ‘artificial pancreas’, or is CGM enough? • Some pediatric patients can successfully achieve A1C values <7% without significant hypoglycemia for many years • Even within a clinical trail, many cannot achieve a significantly lower A1C with CGM, due to the inability to consistently wear the device Why do we need an ‘artificial pancreas’? Current ‘usual’ therapy does not achieve goal A1C values in over 50% of children Using more intensified management can lower A1C levels in many patients The newest current technology - CGMcan also be important in achieving target glycemic levels for some patients Conclusion • An artificial pancreas may remove enough of the ‘human error’ and ‘hassle’ factor to allow more patients to achieve success • A cure for adolescence would also help