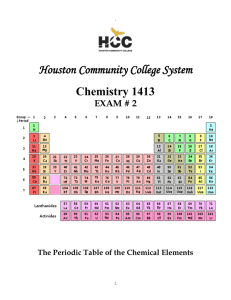
Draw the following Lewis diagrams: N , CH O, CH , H
advertisement

SCH 3U1 POLARITY OF MOLECULES Draw the following Lewis diagrams: N2, CH2O, CH4, H2O Fill in the following chart. Molecule Bond Polarity Molecular Polarity State of Substance Solubility in Water N2 CH2O CH4 H2 O 1. Bond polarity describes the location of the ___________ between two atoms. It may be classified as covalent (equal sharing) and polar covalent (unequal sharing) or ionic (complete transfer). 2. Molecular polarity describes the location of electrons around a neutral molecule. This is due to the ___________ of the atoms. If the electrons are more concentrated at one end of a molecule, it is described as being polar. That is, having a slight positive and negative pole. This slight charge is denoted by the symbol – δ. If there are no areas where electrons are concentrated within a molecule, the molecule is described as being non-polar (no poles). 3. Solid, liquid or gas at room temperature? 4. Is the substance soluble in water (a polar sovent)?