File
advertisement
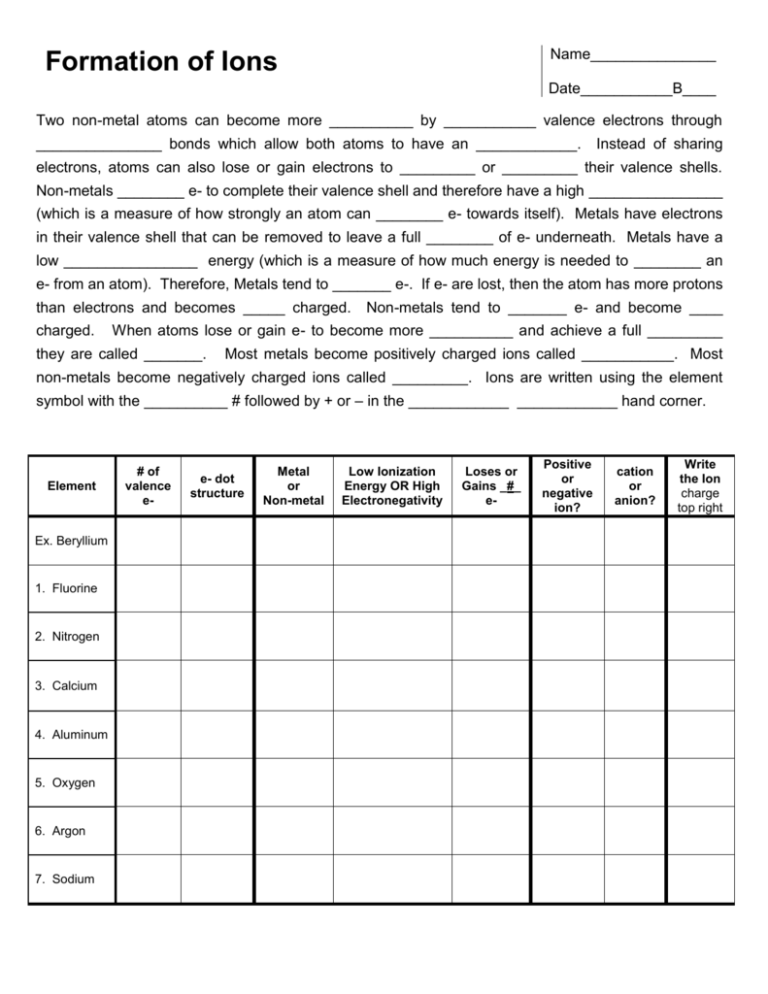
Name_______________ Formation of Ions Date___________B____ Two non-metal atoms can become more __________ by ___________ valence electrons through _______________ bonds which allow both atoms to have an ____________. Instead of sharing electrons, atoms can also lose or gain electrons to _________ or _________ their valence shells. Non-metals ________ e- to complete their valence shell and therefore have a high ________________ (which is a measure of how strongly an atom can ________ e- towards itself). Metals have electrons in their valence shell that can be removed to leave a full ________ of e- underneath. Metals have a low ________________ energy (which is a measure of how much energy is needed to ________ an e- from an atom). Therefore, Metals tend to _______ e-. If e- are lost, then the atom has more protons than electrons and becomes _____ charged. charged. Non-metals tend to _______ e- and become ____ When atoms lose or gain e- to become more __________ and achieve a full _________ they are called _______. Most metals become positively charged ions called ___________. Most non-metals become negatively charged ions called _________. Ions are written using the element symbol with the __________ # followed by + or – in the ____________ ____________ hand corner. Element Ex. Beryllium 1. Fluorine 2. Nitrogen 3. Calcium 4. Aluminum 5. Oxygen 6. Argon 7. Sodium # of valence e- e- dot structure Metal or Non-metal Low Ionization Energy OR High Electronegativity Loses or Gains _#_ e- Positive or negative ion? cation or anion? Write the Ion charge top right Name_______________ Formation of Ions Date___________B____ Complete each statement by filling in the blank and/or circling the answer. 1. The reactivity of elements is based on the number of [ core OR valence ] electrons. 2. The [ Transition Metals OR Noble Gases ] with ___ valence e- have the most stable configuration 3. Metals tend to [ lose OR gain ] and non-metals tend to [ lose OR gain ] . 4. Metals tend to [ lose OR gain ] e- in order to [ fill up OR empty ] their valence shell 5. If an atom loses e-, then it becomes [ + OR - ] charged because it has more protons than e-. 6. If an atom gains e-, then it becomes [ + OR - ] charged because it has less protons than e-. 7. Alkali metals have _____ valence e- and will form ions with [ +1 +2 +3 ] charge. 8. Alkali earth metals have _____ valence e- and will form ions with [ +1 +2 +3 ] charge. 9. Halogens have _____ valence e- and will form ions with [ -3 -2 -1 ] charge. 10. Elements in the Oxygen column have _____ valence e- and will form [ -3 OR -2 ] ions. 11. Elements in the Nitrogen column have _____ valence e- and will form [ -3 OR -2 ] ions. 12. Metals lose e- [ more OR less ] easily than non-metals because they have [ low OR high ] ionization energy, while non-metals gain e- [ more OR less ] easily than metals because they have [ low OR high ] electronegativity. # of Element valence e1. Barium 2. Chlorine 3. Neon 4. Lithium 5. Sulfur 6. Phosphorous 7. Gallium 8. Iodine e- dot Metal or structure Non-metal Low Ionization Energy OR High Electronegativiy Loses or Gains _#_ e- cation or anion? Write Ion









