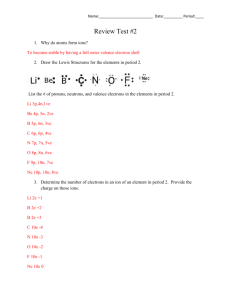
Alien Periodic Table Column 1 – Ch should be at the top, and Q in
advertisement

Alien Periodic Table Column 1 – Ch should be at the top, and Q in period 4. The inert/Noble gases – Bo in period 1, L in period 2, J in period 3, and Wo in period 4. Halogens – Column 17, with V in period 4. Metalloids – Column 13: E in period 2, Yz in period 3 Column 14: Hi in period 3, T in period 4 Column 15: SS in period 4 1. Fill in the blank with the correct element: He Rn Ra N Na ` Cs C W F H S Cl (chlorine) P Sn Hg 2. Lewis Dot structures Ask Mr. Z 3. Why are elements that gain of lose only one electron considered the most reactive? a. They are “desperate” to achieve a full electron shell and will readily react to gain.lose as necessary. 4. Metals – ductile, malleable, conductive, and shiny. Non-metals: nonductile, non malleable, non conductive, and non-shiny (aka dull). 5. Mendeleev first organized by increasing atomic mass. Interpreting the Periodic Table 1. K and F 2. D and R 3. K and F 4. K 5. K, F, D, and R 6. R would have an atomic number of 23 1. 2. 3. 4. Na, 1 valence, metal, group 1, period 3 Mg, 2 valence, metal , group 2, period 3 Al, 3 valence, metal, group 3, period 3 Si, 4 valence, metalloid (semiconductor) group 4, period 3 1. 2. 3. 4. 5. 6. 7. 8. F, groups/families True F, ductile F, metals F, malleable True True F, nonmetals 2. Which of the following represent elements and which compounds O2 – element Ar – element KCl – compound H2 – element Al – element NH3 – compound 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. C, halogen D, one valence electron A, highly reactive B, metals D, semiconductors (metalloids) D, semiconductors A, halogens E, alkali metals C, transition metals B, alkaline earth metals 1. A – alkali metal B – metalloid C – transition D – alkaline earth metal 2. A – nonmetal B – halogen C – noble gas D – nonmetal 1. Draw a helium atom. 2. Valence electrons correlate to the group numbers at the top if you skip the transition metals. 3. Atomic number is the number of protons, while mass number is protons plus neutrons. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 5. B, properties D, none of the above A, 30 A, valence electrons. D, more D, all of the above A, protons A, # of neutrons C, 39 C, both a and b a. similar b. similar c. different d. similar 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. D, scientific basis A, atoms are made of electrons B, neutral C, molecules B, 1 A, move in set paths. C, valence electrons D, elements A, orbitals B, nucleus 3. the further out the electrons are, the more energy they possess. Inner electrons have lower energy than outer electrons.