Solubility and Separation Techniques Test Five Write all answers on
advertisement

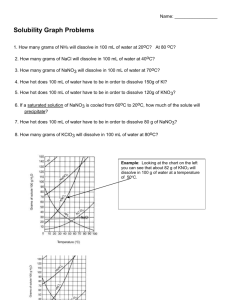
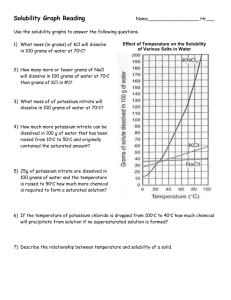
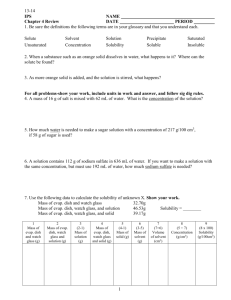
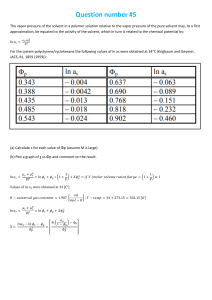
Solubility and Separation Techniques Test Five Write all answers on the test answer sheet. Do not write on this test booklet. For questions 1 -5 refer to the solubility graph. 1. What two pieces of information (besides the grams of solute) are needed to describe the solubility of a substance? 2. How many grams of KNO3 dissolve at 20 degrees in 50 mL of water? 3. Which substance is probably a gas? 4. Which substances’ solubility is most affected by temperature? 5. At what temperature will 20 grams of KClO3 dissolve in 100 mL of water? For questions 6-10 fill in the blank on your answer sheet with the best word or phrase. 6. 7. 8. 9. 10. This is a characteristic property used in centrifuges and flotation. This technique is used to separate alcohol and water This is the term for a substance doing the dissolving This is the term for a measure of the grams of solute in a certain volume of solvent. This is the term for a homogeneous mixture of solute and solvent. 11. When 100 cm3 of methanol (density 0.80 g/cm3) is mixed with 100 cm3 of water (density 1.00 g/cm3), the volume of the mixture is 192 cm3. What is the mass, in grams, of the mixture? a. b. c. d. e. 200 192 180 173 154 12. A student uses a centimeter ruler to measure the edges of a rectangular block of metal, estimating the measurement to the tenth of a millimeter. She obtains 1.25 cm, 1.23 cm, and 1.22 cm. Which is the best was for her to express the volume of the block in cm3? a. b. c. d. e. f. 1.87575 1.876 1.88 1.9 2 13. A 15-cm3 sample of a certain liquid was heated and its temperature was recorded as a function of time. The graph of Figure 1 was plotted from the data. If a completely different substance, with greater intermolecular forces was heated in the same way using the same amounts and the same time frame, which graph would most likely result? Note: Graph C does not plateau…the curve continues to rise at the top. 14. Three waterproof blocks were examined by a student who measured their volumes by dropping them into a graduated cylinder containing water. He then determined the mass of each block on his balance and recorded the data below: Block 1 2 3 Volume (cm3) 20 32 15 Mass (g) 25 40 25 Which blocks could be made of the same material? Explain how you know. 15. In the density of gas lab, you record the data below: Mass of test tube, water, and tablet 37.262g Mass of test tube and remaining contents 36.560g Volume of the gas collected 381.6ml What is the density of the gas? Practice Test Five: Separation and Solubility Name: ______________________ Per: _______ 1. _____________________________________________________________ _______ 2. __________________________ 3. __________________________ 4. __________________________ 5. __________________________ 6. __________________________ 7. __________________________ 8. __________________________ 9. __________________________ 10. ______________________ 11. ______________________ 12. ______________________ 13. ______________________ 14. ______________________ 15. ______________________ Lab Journal Questions Solubility Lab: 1. Question: 2. Hypothesis and rationale: 3. Independent variable: ____________________________________ 4. Dependent variable: ____________________________________ 5. Constants: (list two): ____________________________________ 6. What did you conclude?: _____________________________________________________ _____________________________________________________ _____________________________________________________ _____________________________________________________ ____________________________________________ 7. Your group did a series of experiments about solubility. Based on the results of your group what would you say are the key factors that affect how well a solute dissolves? List three. _____________________________________________________ _____________________________________________________