CH R2 Acids and Bases STUDY GUIDE Science McCloud
advertisement

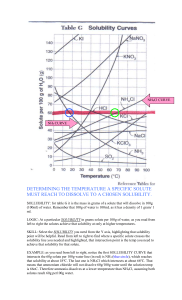
CH R2 Acids and Bases STUDY GUIDE Science McCloud 1. A liquid mixture in which particles can be seen and easily separated by settling or filtration is a? Suspension 2. How is a solute different from a solvent in a solution? The solute is present in a smaller amount 3. How can a scientist safely tell whether an unknown solution is salt in water or sugar in water? By testing the electrical conductivity of the solution 4. Weak tea is an example of a? Dilute solution 5. When you add so much solute that no more dissolves, you have a? Saturated solution 6. If two unidentified solids of the same texture and color have different solubilities in 100 grams of water at 20oC, you could conclude that? They are different substances. 7. What is one way to increase the solubility of sugar in water? Heat the water. 8. How would a solute affect the boiling point of water? The water will boil at a higher temperature. 9. When a solute is added to a solvent, the freezing point of the solution is? Lower than the freezing point of either substance alone. 10. Which is a characteristic property of acids? They turn blue litmus paper red. 11. Acids are described as corrosive because they? Eat away at other materials. 12. Which is a likely use for a base? Making soaps and detergents. 13. In a water solution, how do acids differ from bases? Acids form hydrogen ions (H+), while bases form hydroxide ions (OH-). 14. In water, bases form? Hydroxide ions. 15. The pH scale measures? The concentration of hydrogen ions. 16. If you have a solution of a strong acid and a solution of a weak acid of equal concentration and volume, then the? Strong acid will have a higher pH. 17. Neutralization is a reaction between a? Acid and a base. 18. What does a neutralization reaction produce? Water and a salt. 19. The process that breaks down complex molecule of food into smaller particles is called? Digestion 20. How does chemical digestion differ from mechanical digestion? 21. How is pH important during digestion? Different enzymes work best at different pH values. 22. Compared to the pH of saliva, the pH of stomach juices is? Lower. 23. Normal rainfall is slightly acidic, which means its pH must be? Between 5 and 7 24. When a few spoonfuls of sugar are mixed into a cup of water, sugar is the? Solute 25. When a solid compound dissolves in water? Each of its particles becomes surrounded by water molecules. 26. A measure of how well a solute can dissolve in a solvent at a given temperature is that substance’s? Solubility. 27. What does it mean is a compound has a solubility of 15 g in 100 g of water at 0oC? 15 g of the compound will dissolve in 100 g of water at 0oC. 28. A mixture containing particles that are too small to bee seen easily but are large enough to scatter a light beam is called a? Colloid. 29. Acids naturally present in food are safe to eat because they usually are? Weak. 30. You can find the pH of a substance by using? Litmus indicator. Short Answer Use the diagram to answer each question. 31. What happens to the solubility of potassium nitrate (KNO3) as the temperature rises? The solubility increases. 32. According to the graph, which of the compounds is most soluble at 0°C? Which is least soluble at 100°C? KI is most soluble at 0oC, and NH3 is least soluble at 100oC. 33. Compare the solubility of sodium nitrate (NaNO3) to that of ammonia (NH3). Ammonia (NH3) is more soluble than sodium nitrate (NaNO3) at low temperatures and less soluble than sodium nitrate (NaNO3) at high temperatures. 34. About how many grams of potassium nitrate (KNO3) will settle out when a saturated solution made in 100 grams of water is cooled from 70°C to 50°C? 130 g – 80 g = 50 g 35. Above 70°C, what other compounds besides ammonia (NH3) have a lower solubility than that of potassium nitrate (KNO3)? Sodium nitrate (NaNO3) and ammonium chloride (NH4Cl) 36. At 70°C, 60 grams of ammonium chloride (NH4Cl) are added to 100 grams of water. What will happen if 10 grams more of ammonium chloride are added to the solution? Explain. Most of the additional ammonium chloride will fall to the bottom of the container because the solution will be saturated. At 70 oC, the solubility of ammonium choloride in 100 grams of water is just over 60 grams. Use the diagram to answer each question. 37. Use the pH scale to compare an apple and a lemon. Lemons have a pH of about 2 and apples have a pH of about 3. Lemons are more acidic. 38. In terms of pH, explain what would happen if you mixed vinegar with a solution of ammonia. What type of products would form? Vinegar is an acid and ammonia is a base, so a neutralization reaction would occur. The pH would be closer to 7 than either vinegar or ammonia alone. A salt and water would be produced. 39. What does the pH of drain cleaner tell you about the dangers of such a product? A high pH means that drain cleaner is very basic. The solution can burn and damage skin. 40. What color would litmus paper turn in a solution of baking soda? Explain your answer in terms of pH. Blue. Baking soda has a pH of about 8.5, which means it is a base. Bases turn red litmus paper blue. 41. Why would you expect soap to taste bitter? Soap is a base, and bases taste bitter. 42. What does the pH of water tell you about how the water differs from the other items shown in the diagram? Water has a pH of 7, which is neutral. It is not an acid or a base.