Solubility Graph Problems
advertisement
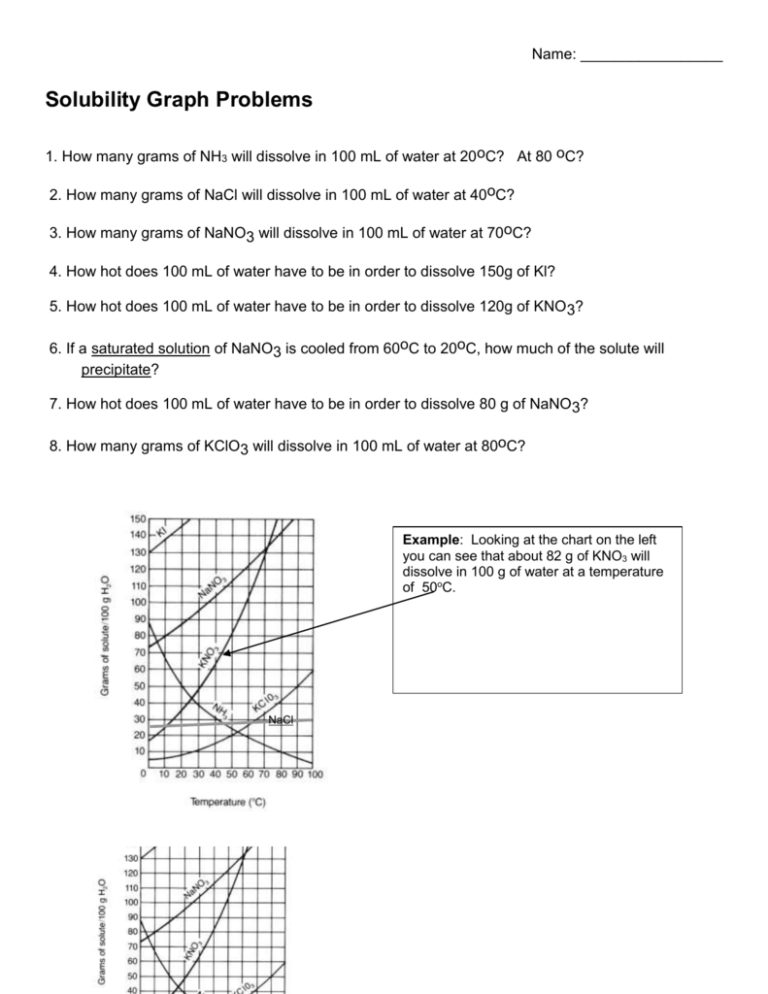
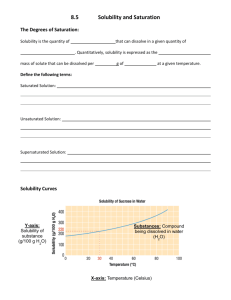
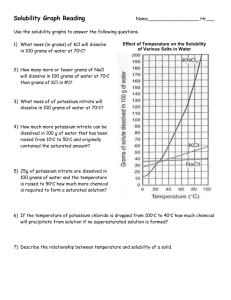
Name: _________________ Solubility Graph Problems 1. How many grams of NH3 will dissolve in 100 mL of water at 20oC? At 80 oC? 2. How many grams of NaCl will dissolve in 100 mL of water at 40oC? 3. How many grams of NaNO3 will dissolve in 100 mL of water at 70oC? 4. How hot does 100 mL of water have to be in order to dissolve 150g of Kl? 5. How hot does 100 mL of water have to be in order to dissolve 120g of KNO 3? 6. If a saturated solution of NaNO3 is cooled from 60oC to 20oC, how much of the solute will precipitate? 7. How hot does 100 mL of water have to be in order to dissolve 80 g of NaNO3? 8. How many grams of KClO3 will dissolve in 100 mL of water at 80oC? Example: Looking at the chart on the left you can see that about 82 g of KNO3 will dissolve in 100 g of water at a temperature of 50oC. NaCl Name: _________________ Solubility Graphing 1. Why do the temperatures on the graph only go from 0º C to 100º C? 2. Which substance has a solubility of 25 g/100 cm3 of water at a temperature of about 13ºC? 3. What is the solubility of potassium iodide at 30ºC? 4. At what temperature does potassium nitrate have a solubility of 138 g/100 cm3 of water? 5. What is the range of temperatures for when sodium chloride has a solubility of 40 g/100 cm3 or higher? 6. Which substance is most soluble at 60º C? 7. Which two substances have the same solubility at 80º C? 8. Which substance's solubility changes the most from 0º C to 100º C? 9. Which substance's solubility changes the least from 0º C to 100º C? 10. What is the solubility of potassium nitrate at 90º C? 11. At what temperature does potassium iodide have a solubility of 150 g/ 100 cm3 water? 12. You have a solution of potassium chlorate containing 4 g at 65º C. How many additional grams of solute must be added to it, to make the solution saturated?