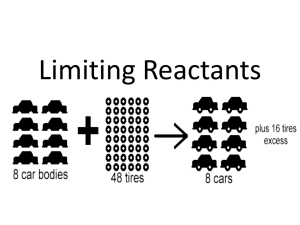
Brian Dempsey, Simon Yang Limiting Reactant and Percent Yield
advertisement

Brian Dempsey, Simon Yang Limiting Reactant and Percent Yield Limiting Reactant: the reactant that limits the amount of product that can be formed in a chemical reaction Excess reactant: the substance that is not completely used up in a reaction Steps to determining limiting reactant: 1. Balance the equation. 2. Pick a reactant. Convert grams of one reactant into grams of the second reactant. 3. Compare the amount given in the reaction to the amount calculated. a. If the number is smaller, then the second reactant is in excess. b. If the number is larger, then the second reactant is limiting. Theoretical Yield: the amount of product that would result if all the limiting reactant yielded Actual Yield: the amount of product that was actually obtained Practice Problems 1. Silver nitrate and sodium chloride react with each other to create silver chloride and sodium nitrate (AgNO3 + NaCl → AgCl + NaNO3). If we initially started out with 10 grams of silver nitrate and 15 grams of sodium chloride, how many grams of sodium nitrate can you make? 2. Hydrogen and Oxygen react with each other to create water. 2H2(g) + O2(g) 2H2O The density of O2 is 1.429 g/L and the density of H2 is 0.08988 g/L. How much water, in grams, will be produced from this reaction if you have 42.0 L of H2 and 20.0 L of O2? Which reactant is the limiting reactant? How much of the excess reactant is left over after the reaction? 3. If you have 5.00 g MgCl2, how many grams of CaOH2 are required to fully react with the Magnesium Chloride? MgCl2(aq) + Ca(OH)2(aq) Mg(OH)2(s) + CaCl2(aq) How many grams of Mg(OH)2 will be formed by this reaction? What is the percent yield if you performed this reaction and obtained 2.00 g Mg(OH)2? 4. What is the percent yield of the reaction if 40.00 kg of CaF2 was treated with an excess of H2SO4 and yields 19.07 kg of HF? (Formula: CaF2 + H2SO4 -> CaSO4 + 2 HF) 5. Barium sulfate is produced by reacting Barium sulfide with sulfuric acid. BaS(s) + H2SO4(aq) BaSO4(s) + H2S (aq) What mass of BaSO4 will be produced from the reaction of 75.0 g BaS and 40.0 g H2SO4? What is the percent yield of the reaction if a scientist obtains 30.0. g BaSO4? 6. Limiting ReactantIf you run a reaction between potassium chloride and sulfuric acid, potassium sulfate will be formed. 2KCl(aq) + H2SO4(aq)2HCl(aq) + K2SO4(aq) With 10.0 g KCl and 35.0 g H2SO4, what will your limiting reactant be? How many grams of K2SO4 and HCl will be formed? How much of the excess reactant will be left over? 7. To remove rust from steel, the rusted steel is placed in HCl. Fe2O3(s) + Fe(s) + 6HCl(aq) 3FeCl2(aq) + 3H2O(l) If you have 100.00 g Fe2O3, an excess of Fe, and 37.0 g HCl, how much FeCl2 will be created? 8. Harmful gases can be reduced from the emissions of coal burning using NaOH. 2NaOH(s) + CO2(g) Na2CO3(aq) + H2O(l) How many grams of Na2CO3 will be produced if you have 47.0 g NaOH and 34.0 g CO2? How much of the excess reactant will you have left over at the end of the reaction? 9. What is the mass in grams of oxygen produced if 626.00g of lithium perchlorate are heated and allowed to decompose? 10. The Ostwald process for producing nitric acid from ammonia consists of the following steps. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) 4 NO(g) + 2 O2(g) 4 NO2(g) 3 NO2(g) + H2O(g) 2 HNO3(aq) + NO(g) If the yield in each step is 93.6%, how many grams of nitric acid can be produced from 3.4 kg of ammonia?