Chemistry Semester 2 Ms. Boon Unit 9: Reaction Rates and
advertisement

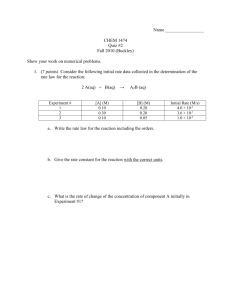
Chemistry Semester 2 Ms. Boon Unit 9: Reaction Rates and Equilibrium Homework #1 pp. 585 #1-13 1. What does the word “rate” mean in everyday life, and what do chemists mean by “reaction rate”? A: A rate is a change in some quantity divided by the length of time over which the change occurred. A reaction rate is the decrease in the concentration of a reactant (or the increase in the concentration of a product) during a small time interval, divided by the duration of that interval and by the corresponding coefficient in the chemical equation. 2. What is the name given to the branch of chemistry dealing with reaction rates? What are such studies important? A: Chemical kinetics is the branch of chemistry dealing with reaction rates. Kinetics helps chemists learn about the mechanism by which a reaction occurs. 3. Why is a collision between molecules necessary in many reactions? A: Atoms must be in contact with each other for new bonds to form. 4. How may reaction rates by measured? A: Reaction rates may be measured by measuring the change in the concentration of a reactant or product during a short time interval and thereby calculating the rate. 5. Explain why reactant concentration influences the rate of a chemical reaction. A: The greater the concentration, the more molecules of reactant there are to react and therefore the greater the reaction rate. 6. Give examples of the strong effect that temperature has on chemical reactions. A: Many reactions are temperature dependent. They speed up when heat is added. For example, chemical reactions in the human body increase when we get a temperature. That is why we need to maintain our core body temperature. Food molds faster at higher temperatures. Organic substances decompose faster at higher temperatures. 7. What is unique about surface reactions? A: The rate of a surface reaction is proportional to the area of the surface. 8. Why must coefficient be included in the definition of reaction rate? a. The rates of concentration change, for the species that participate in the reaction, are proportional to that species’ coefficient in the chemical equation. Therefore, in order that the rate of reaction be defined in a way that is independent of the choice of species, the rate of concentration change must be divided by the corresponding coefficient. 9. Calculating the reaction rate from a product appeared to give an answer different from that calculated from a reactant. Why? a. One possibility is that the product might be involved in some other reaction, a side reaction. 10. The usual unit for reaction rate is M/s. Can you think of another unit? a. Moles per cubic centimeter per minute is one possible unit. Grams per second could work too. 11. Explain why an increase in the frequency of collisions is not an adequate explanation of the effect of temperature on reaction rate. a. There is only a very mild increase in collision rate with temperature. But, the reaction rate increases massively. 12. Would the factors that affect the rate of a chemical reaction influence a physical change in the same way? Explain. a. Generally, yes. For example, an increase in temperature greatly increases the rate of evaporation of a liquid. 13. Why does pressure affect the rates of gas reactions? a. Pressure affects the rate of gas reactions because it changes the concentration of the reactants.