limiting reactants
advertisement

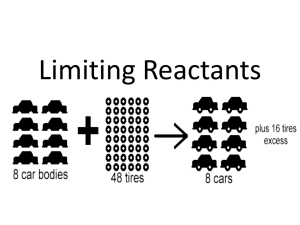
Chemistry 139 Reminders for the rest of the homework: when you are given mass of more than one starting material you must calculate the amount of a product (the same product!) based on the starting materials for each reactant the limiting reagent is NOT necessarily the reactant present in the smallest amount, it is the reactant that produces the smallest amount of product. Therefore, you cannot simply examine the amounts of reactants and pick the limiting reagent – you must perform calculations! the general set-up: grams A moles A (using the molar mass of A) mole:mole ratio moles B grams B (using the molar mass of B) you may use a different method than the one listed in class if you choose- but you better be sure it’s a good method! The problems in this homework will ask you to follow the method shown in class – if you are using a different method, make sure you indicate the following: the smallest amount of product formed and the limiting reagent/reactant Steps that we used in class to determine the theoretical yield (the theoretical amount of product that can be formed, and to identify the limiting reactant Step 1: balance the reaction Step 2: circle the product that you will be calculating the amount of (you only got one in this reaction) Step 3: calculation the molar masses (mm) of all species that you are interested in (asked about!) in the problem Step 4: start with the amounts of each reactant and calculate the amount of product that you circled that could be formed Step 5: circle the smallest number (of the amount of product that can be formed) – label this as your theoretical yield Step 6: draw an arrow from the circled product to the reactant used to get that number – label that as your limiting reactant Chemistry 139 Name: _______________________________ Homework 6 Calculations involving reaction stoichiometry: the mole:mole ratio 1. Solid iron metal reacts with oxygen gas to give iron (III) oxide. ______Fe (s) mm = + ______O2 (g) mm = _______ Fe2O3 (s) mm = If you react 4.88 g of iron with 2.68 grams of O2 - what is the theoretical yield of iron (III) oxide that can be produced? Which species is the limiting reactant? Show calculations here: Limiting reactant: ______________________ Theoretical Yield: ________________________ 2. Nitrogen gas can be prepared by passing gaseous ammonia over solid copper (II) oxide at high temperatures, as described by the following balanced equation. 2 NH3(g) mm = + 3 CuO(s) mm = 1N2(g) + 3 Cu(s) + 3 H2O(g) mm = How many grams of N2 are formed when 18.1 g of NH3 are reacted with 90.4 g of CuO? Which species is the limiting reactant? Calculations: Limiting reactant: _____________________ Theoretical Yield: ________________________ Chemistry 139 3. Chlorine dioxide is used as a disinfectant and bleaching agent. In water, it reacts to form chloric acid (HClO3), according to the following balanced equation: 6 ClO2 mm = + 3 H2O mm = 5 HClO3 + HCl mm = If 142.0 g of ClO2 are mixed with 38.0 g of H2O, how many grams of HClO3 are formed? Which species is the limiting reactant? Calculations: Limiting Reactant: _________________________ Theoretical Yield: ____________________________ 4. The deep blue Cu(NH3)4SO4 is made by the reaction of copper (II) sulfate with ammonia, as described by the following reaction: _______CuSO4(aq) + _______ NH3aq) _______Cu(NH3)4SO4(aq) mm = mm = mm = If you use 10.0 grams of CuSO4 and 10.0 grams of NH3, what is the theoretical yield for Cu(NH3)4SO4? Which species is the limiting reactant? Calculations: Limiting Reactant: _________________________ Theoretical Yield: ____________________________ Chemistry 139 Using a similar format as above – answer the following questions: insert spaces and solve each! 5. Nitrogen monoxide (g) and hydrogen gas react to form NH3 (g) and water (g). Write the balanced chemical reaction with phases for this reaction Starting with 86.3 grams of nitrogen monoxide and 25.6 grams of hydrogen gas, determine the theoretical yield of NH3 in grams Identify the limiting reactant Determine the grams of leftover/excess reactant 6. Use the following reaction to answer the next set of questions: _____TiO2 (s) + _____C (s) → _____Ti (s) + _____CO (g) balance the following reaction When 28.6 grams of C is allowed to react with 88.2 grams of TiO2, how many grams of Ti (s) can be produced? Identify the limiting reactant Determine the grams of leftover/excess reactant