Hybridisation - 12S7F-note
advertisement
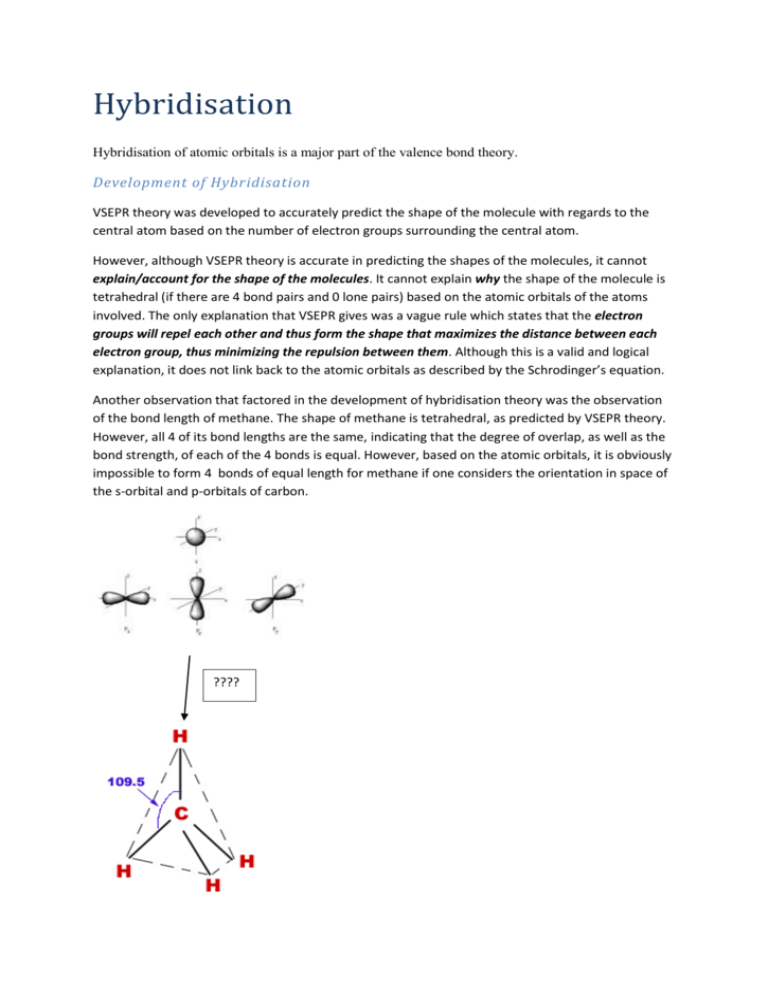
Hybridisation Hybridisation of atomic orbitals is a major part of the valence bond theory. Development of Hybridisation VSEPR theory was developed to accurately predict the shape of the molecule with regards to the central atom based on the number of electron groups surrounding the central atom. However, although VSEPR theory is accurate in predicting the shapes of the molecules, it cannot explain/account for the shape of the molecules. It cannot explain why the shape of the molecule is tetrahedral (if there are 4 bond pairs and 0 lone pairs) based on the atomic orbitals of the atoms involved. The only explanation that VSEPR gives was a vague rule which states that the electron groups will repel each other and thus form the shape that maximizes the distance between each electron group, thus minimizing the repulsion between them. Although this is a valid and logical explanation, it does not link back to the atomic orbitals as described by the Schrodinger’s equation. Another observation that factored in the development of hybridisation theory was the observation of the bond length of methane. The shape of methane is tetrahedral, as predicted by VSEPR theory. However, all 4 of its bond lengths are the same, indicating that the degree of overlap, as well as the bond strength, of each of the 4 bonds is equal. However, based on the atomic orbitals, it is obviously impossible to form 4 bonds of equal length for methane if one considers the orientation in space of the s-orbital and p-orbitals of carbon. ???? Due to these inadequacies of current explanations, scientist Linus Pauling developed the hybridisation theory. Details of Hybridisation Theory Pauling proposed that in methane, the 2s-orbital and the 3 2p-orbitals hybridise and form 4 sp3 hybrid orbitals arranged in a tetrahedral shape. These sp3-orbitals are also degenerate, which means that they all have the same energy level. Similarly, the other shapes predicted by VSEPR theory also have their own hybrid orbitals to account for their shapes: trigonal planar by 3 sp2-orbitals; Linear triple molecules by 2 sp-orbitals; trigonal bipyramidal by 5 sp3d-orbitals and octahedral by 6 sp3d2orbitals. Note that the number of orbitals is conserved when orbitals hybridise, i.e. the number of atomic orbitals that hybridise is equal to the number of hybrid orbitals produced. Also note that all hybrid orbitals are degenerate, i.e. all hybrid orbitals formed have the same energy level. Also note that hybrid orbitals have an energy level in between the energy levels of the original atomic orbitals. For example, the energy level of the sp3-orbital is higher than the energy level of the s-orbital but lower that the energy level of the p-orbital. Hybrid orbitals have character of all parent atomic orbitals. However, the character of these parent atomic orbitals are not equal within each set of hybrid orbitals. For example, the sp3-orbital has more p-character and less s-character than sp2 or sp-orbital, by virtue of the ratio of the parent orbitals. More s-character typically means that the energy level of the hybrid orbital is closer to the energy level of the parent s-orbital rather than the energy level of the p-orbital, vice versa for pcharacter. Also, note that one atom can only have 1 hybridisation scheme at any one time, but the hybridisation scheme of the atom can change during a chemical reaction. Hybridisation is thus able to provide a way how the s, p, d… orbitals can produce the other ‘random’ shapes of molecules. Common Questions about Hybridisation theory One of the most common questions asked about hybridisation is how do atoms ‘know’ whether to form (for example) sp3 or sp2 hybrid orbitals. Many students believe that in the central atom, the hybrid orbitals form first from the atomic orbitals, then the other atoms form a covalent bond with the hybrid orbitals. Because a different hybridisation scheme will cause the product molecule formed to be different, the atom appears to know what molecule is it going to be, forming the relevant hybridisation; all before the atom actually reacts. Thus from this assumption, it is illogical to say that the atom ‘knows’ which hybridisation to get into in order to form the compound even before the reaction takes place. The actual explanation for this is actually in the development of hybridisation theory. The hybridisation theory is actually an explanation for the shape of the molecules. The shapes come first, and the explanation comes later. Thus, it can be said that the molecule is formed first, the hybridisation comes later. However, this explanation, while true, is slightly abstract and confusing. A better explanation is as below: One can imagine the hypothetical formation of methane. In volume of space, there are gaseous monoatomic carbon and monoatomic hydrogen colliding with each other to form methane. Initially, there is no hybridisation scheme in the carbon atom, the electrons in the carbon atom resides in their normal s and p-orbitals. In an instance, when 4 hydrogen atoms approaches the carbon atom at the correct tetrahedral geometry, the electrostatic forces due to the electron cloud and the nucleus of the hydrogen atoms at the correct geometry would induce (attract/repel) the electron cloud (s and p-orbitals) of the carbon atom to form the sp3-orbital shape. As the hydrogen atoms come closer, the s- and p-orbitals of the carbon atom to be more and more distorted until when the hydrogen atoms reaches 110 pm from the carbon atom, the full sp3-hybrid orbital is formed from the 2s-orbital and the 3 2p-orbitals. Therefore, it is the approach of the other atoms that induces the hybridisation and the hybridisation forms at the same time as the molecule is formed. Note that the above reaction stated is strictly hypothetical. It probably does not exist. It is only dreamed up of me in an attempt to rationalise the apparent paradox where the atom appears to know which molecule it is going to form before the reaction takes place.









