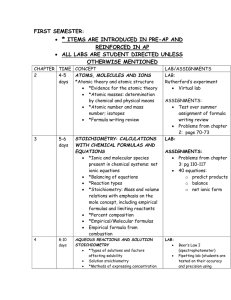
LIST OF TOPICS COVERED DURING THIS COURSE
advertisement

LIST OF TOPICS COVERED DURING THIS COURSE The following should serve as a checklist for your notebook. The topics below include all topics that have been covered this semester and are testable on your final exam. These topics should be studied from a variety of source including inclass notes, homework questions, lab questions, assignments, and in-class discussions. If you missed any notes, please get the information from a classmate, or from myself. UNIT 1 Atomic mass Atomic number Particles of an atom (electrons, protons, neutrons) Ions (cations, anions) Multivalent ions Polyatomic ions Ions in the body Isotopes Isotopic abundance Average atomic mass Radiation and radioisotopes Periodic table 4 main groups of the periodic table (and characteristics) periodic trends (atomic radius, ionic radius, ionization energy, electron affinity, electronegativity) review of Bohr-Rutherford diagram ionic compounds (properties, formation, structure, naming, and bonding) molecular element molecular compound (properties, drawing, bonding, naming) nomenclature (ionic, molecular, acids and bases) hydrogen bonding (and how it relates to special properties of water) UNIT 2 Evidence of a chemical reaction Catalysts 4 main kinds of reactions (synthesis, decomposition, single displacement, double displacement) neutralization (with hydroxide and carbonate) combustion (complete and incomplete) elements and their oxides (basic and acidic) balancing equations UNIT 3 the mole (Avogadro’s number, mole equation) significant digits calculating molar mass of compounds calculating number of particles/atoms mole ratios percentage composition law of definite proportions empirical formulas molecular formulas stoichiometry limiting reactants actual yield theoretical yield percent yield UNIT 4 importance of water hard water vs. soft water types of contaminants solution components (solute and solvent) Dilute vs. concentrated solutions Heterogeneous mixture Hydration Dissociation Miscible and immiscible Surfactants Degrees of saturation Solubility curves Pressure Total ionic equations Net ionic equations (spectator ions) Stoichiometry of solutions Acids (definition, characteristics, naming) Bases (definition, characteristics, naming) Titration UNIT 5 KMT Atmosphere STP and SATP Absolute zero Converting from absolute temp to Celsius Charles’ law Boyle’s law Gay-Lussac’s law Combined gas law Avogadro’s law Molar volume Ideal gas law Law of partial pressures Gas stoichiometry