Physics of Auger electron spectroscopy
advertisement
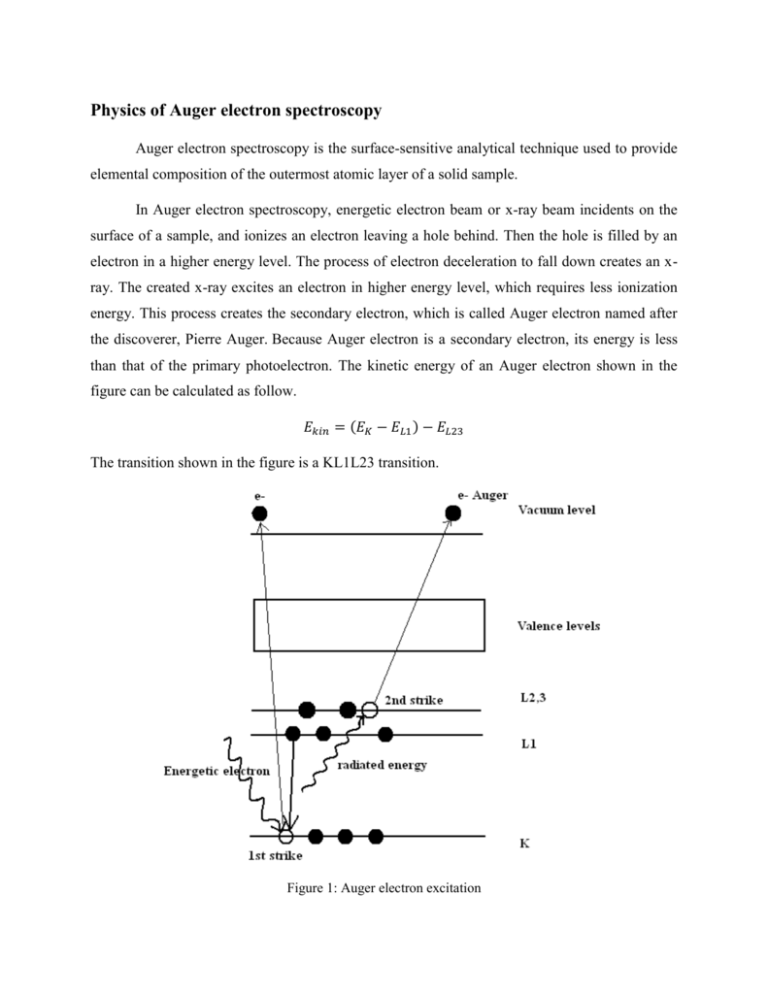
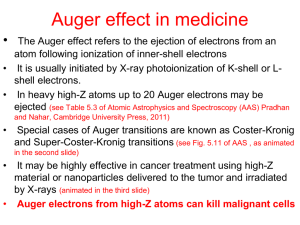
Physics of Auger electron spectroscopy Auger electron spectroscopy is the surface-sensitive analytical technique used to provide elemental composition of the outermost atomic layer of a solid sample. In Auger electron spectroscopy, energetic electron beam or x-ray beam incidents on the surface of a sample, and ionizes an electron leaving a hole behind. Then the hole is filled by an electron in a higher energy level. The process of electron deceleration to fall down creates an xray. The created x-ray excites an electron in higher energy level, which requires less ionization energy. This process creates the secondary electron, which is called Auger electron named after the discoverer, Pierre Auger. Because Auger electron is a secondary electron, its energy is less than that of the primary photoelectron. The kinetic energy of an Auger electron shown in the figure can be calculated as follow. 𝐸𝑘𝑖𝑛 = (𝐸𝐾 − 𝐸𝐿1 ) − 𝐸𝐿23 The transition shown in the figure is a KL1L23 transition. Figure 1: Auger electron excitation Figure 2 is the Auger electron spectra. The electron energy distribution N(E) was obtained by 1-keV primary electron beam incident on a pure silver specimen, the lowest line in the figure. The peak at 1000 eV is the elastically backscattered electrons. The intensity at lower energies corresponds to electrons that are backscattered and have undergone nondiscrete energy losses. The peak in the very low energy region (0 to 50 eV) corresponds to the true secondary electrons. Because we can see nothing from this distribution, we multiply the whole distribution by the factor of 10 shown in the middle distribution. There is a little peak at about 400 eV. This one is the Auger electron peak. It is still too small to identify. We find the derivative of the amplified one; the distribution is shown in the topmost distribution. The peak-to-peak heights (measured from the most positive to the most negative excursion) in the dN/dE spectra and the peak areas under the N(E) curve were shown to be proportional to the number of atoms originating the Auger electrons. These measurements are commonly used to quantify Auger spectra. Because Auger electrons carry small amount of energy, compared to x-ray, its escaped depth is small, so it suits for surface of the sample analysis. Figure 2: auger electron energy distribution Example of Auger electron spectroscopy This example is obtained from A.Kumar, F.Singh, Govind, A.M.Shivaprasad, D.K.Avasthi, and J.C.Pivin (2008). Applied Surface Science. X-ray photoelectron and X-ray Auger electron spectroscopy studies of heavy ion irradiated C60 films, 254:7280-7284. Sample preparation Fullerene thin films were deposited on Si(100) substrates in a vacuum of 1x10 -6 Torr by resistive heating, using commercially available C60 (Aldrich) in a Ta boat. The thickness of the films measured by a quartz crystal thickness monitor was about 500 nm. The preparation steps are as follows. 1. Prepare high vacuum chamber <10-6 Torr using turbo molecular pump 2. Irradiate the films with 200 MeV Au+16 ion beam at different fluencies of 3x1010, 3x1011, 3x1012, and 1x1013 ions/cm2 , using normal incident, room temperature and 0.5 pnA (1 pnA=6.25x109 ions/s). 3. Scan the sample using ion beam in area of 1 cm2 by electromagnetic scanner. 4. Deposit the Au ions about 16.2 keV by electronic energy loss and about 109.4 eV/nm by nuclear energy loss. Experimental procedure The X-ray source, Al Kα (1486.6 eV), was used to produced x-ray in XAES. The spectrometer is operated under a base pressure of 6x10-10 Torr. The XAES was used to quantify different hybridization states. Result XEAS study shows the transformation of sp2 to sp3 hybridization state. The hybridization of C60 is different from the other form of carbon (diamond, graphite and nanotube), as it is neither pure sp2 nor sp3. It is a mixture of s orbital and pz orbital due to the deviation from planarity. The average bond is sp2.278. The N(E) spectra of C60 and irradiate C60 have no significant changes, but dN/dE spectra show the difference, as shown in figure 3. dN/dE spectra are often characterized by the energy separation between the two major excursions and for carbon specimen, which indicate whether the carbon is involved in sp2 or sp3 bonding. Analysis The method to calculate the sp2 percentage is 𝑠𝑝2 % = 𝐷𝑓𝑢𝑙𝑙𝑒𝑟𝑒𝑛𝑒 − 𝐷𝑑𝑖𝑎𝑚𝑜𝑛𝑑 × 100 𝐷𝑔𝑟𝑎𝑝ℎ𝑒𝑛𝑒 − 𝐷𝑑𝑖𝑎𝑚𝑜𝑛𝑑 The 𝐷𝑓𝑢𝑙𝑙𝑒𝑟𝑒𝑛𝑒 corresponds to the distance between the maximum and the minimum in the derivative of XAES spectra. The values obtained at higher fluence are close to the D values obtained in diamond like carbon films with predominantly sp3 bonding. The analysis shows that the sp2 percentage are approximately 81, 34, 11 and 3% for C60 and irradiated films at ion fluence of 3x1010, 3x1012 and 1x1013 ions/cm2, respectively. The discrepancy can be understood as (i) in present experiment, they are probing only surface region, which may be different from bulk, (ii) they are dealing with 16+ charge state, which is less than equilibrium charge state, so there may be some differences in surface properties and bulk properties, (iii) the observed some oxygen contents in C60 film, which increased after irradiation up to few percentage, which may influence the derived sp2 contents. The AXES shows that we can tune the bonding structure of carbon atoms by controlled irradiation of fullerene films. Figure 3: (a) N(E) and (b) derivative 𝑑𝑁 𝑑𝐸 spectra studied by XAES of C60 and ion irradiated films References B.L.Adam et all. (1992), ASM Metals Handbook, Material Characterization, 10 D.P.Woodpuff and T. Delchar (1994), Modern Techniques of Surface Science, Cambridge University press. A.Kumar, F.Singh, Govind, A.M.Shivaprasad, D.K.Avasthi, and J.C.Pivin (2008). Applied Surface Science. X-ray photoelectron and X-ray Auger electron spectroscopy studies of heavy ion irradiated C60 films, 254:7280-7284.