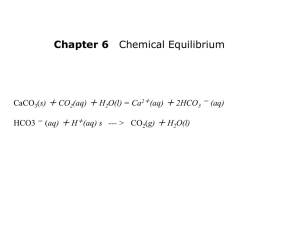
SA HL AcidBase topic 8 18 HL short answer

IB Chemistry 2 – Topics 8 and 18 Acids and Bases
HL – Test 2015
Name:________________________
Short Answer: Please answer in the box provided. If you need to use more space, indicate so in the box and finish your response, clearly marked on the additional paper provided.
/33
1.
Determine the pH of the solution resulting when 200 cm
3
of 0.050 mol dm
–3
HCl(aq) is mixed with 300 cm
3
of 0.10 mol dm
–3
NaOH(aq). (5)
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
2.
Salts may form neutral, acidic or alkaline solutions when dissolved in water.
(i) Explain why a solution of sodium chloride is neutral but sodium carbonate forms an alkaline solution when it dissolves in water. (2)
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
3.
IB Chemistry 2 – Topics 8 and 18 Acids and Bases
HL – Test 2015
(i) Describe how you would make a buffer solution with 2.00 × 10
–2
mol dm
–3
hydrochloric acid solution and 2.00 × 10
–2
mol dm
–3
aqueous ammonia solution. (1)
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
(ii) Identify the base and conjugate acid ions that make up the buffer in (i) (1)
................................................................................................................................................
................................................................................................................................................
................................................................................................................................................
................................................................................................................................................
(iii) State what is meant by a buffer solution. (1)
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
(iv) Describe how the buffer minimizes the effect of the addition of a strong base,
OH
–
(2)
................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
IB Chemistry 2 – Topics 8 and 18 Acids and Bases
HL – Test 2015
(iii) Describe how the buffer minimizes the effect of the addition of a strong acid, H
+
(aq), to the buffer. Illustrate your answer with an ionic equation. (2)
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
.....................................................................................................................................................................................
4.
(a) The chlorate ion is present in chloric acid, HClO
3
, which is a strong acid. The chlorite ion is present in chlorous acid, HClO
2
, which is a weak acid.
(i) Deduce the expression for the ionization constant, K a
, of chlorous acid and calculate its value if the p K a
is 1.96. (2)
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
(ii) Use your answer from part (a) (i) to calculate the [H
+
] and the pH of an aqueous solution of chlorous acid of concentration 0.0531 mol dm
–3
. State one assumption made in arriving at your answer.
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
(4)
(3)
IB Chemistry 2 – Topics 8 and 18 Acids and Bases
HL – Test 2015
(b) A small piece of zinc is added to solutions of chloric acid and chlorous acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids. (2)
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
(c) The graph below shows how the conductivity of the two acids changes with concentration.
250
200
150
100
50
0
Comparision of Chloric and Chlorous acids' conductivity relative to thier concentration
Acid 1
Acid 2
0 0,1 0,2 0,3 0,4
Concentration of Acid in M
0,5
Identify Acid 1 and explain your choice.
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
(2)
IB Chemistry 2 – Topics 8 and 18 Acids and Bases
HL – Test 2015
(d) Weak acids in the environment may cause damage. Identify a weak acid in the environment due to human causes, be sure to discuss the source of the acid and outline one of its effects. (3)
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
5.
(a) The p K a
value for propanoic acid ( CH
3
COOH) is given in Table 21 of the Data Booklet. The graph below shows a computer simulation of a titration of 25.0 cm
3
of 0.100 mol dm
–3
hydrochloric acid with 0.100 mol dm
–3
sodium hydroxide and the pH range of phenol red indicator.
Sketch the graph that would be obtained for the titration of 25.0 cm
3
of 0.100 mol dm
–3 propanoic acid ( CH
3
COOH) with 0.100 mol dm
–3
potassium hydroxide (3)
IB Chemistry 2 – Topics 8 and 18 Acids and Bases
HL – Test 2015
(i) On the graph, be sure to identify the volume of KOH(aq) used and the pH at the equivalence point. [1]
(ii) Using Table 22 in your data booklet, choose a suitable indicator for the reaction in your graph and indicate the color of the indicator at beginning and the end of the titration. [2]
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
....................................................................................................................................................
End of Test.
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................
................................................................................................................................................................................................