Acid-Base Titration and pH
advertisement

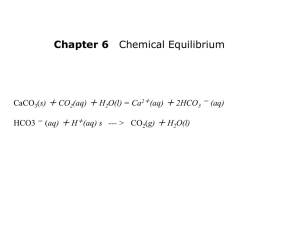
1 Objectives: Chemistry 30 Chapter 15 - Acids and Bases 1. Be able to list the general characteristics of acids and bases (15-1). 2. Name some common binary acid and oxyacids, given their chemical formulas. (15-1) 3. List five acids commonly used in industry and the laboratory, and give two properties of each. (15-1) 4. Define acid and base according to Arrhenius’s theory of ionisation. (15-1). 5. Explain the difference between strong and weak acids and bases. (15-1) 6. Contrast between concentrated and dilute acids and bases (notes). 7. Be able to define acids and bases in terms of the Bronsted-Lowry theory (15-2). 8. Using Bronsted-Lowry theory, identify the acid and the base in chemical reactions (15-2). 9. Using Bronsted-Lowry theory, predict the products of a given acid-base reaction (15-2) 10. Define a Lewis acid and a Lewis base. (15-2) 11. Be able to identify acid-base conjugate pairs (15-3). 12. Using a Ka table, be able to write the acid-base reaction for any given pair of acids. (15-2) 13. Explain the process of neutralization. (15-3) 14. Explain how acid rain can damage marble structures. (15-3) Chapter 16 - Acid-Base Titration and pH 1. Understand the basis of the Kw equation (16-1). 2. Use the Kw equation to determine the hydronium ion and hydroxyl ion concentration of a solution (16-1). 3. Discuss the nature of pH determinations in terms of a logarithmic scale and hydronium ion concentration (16-1). 4. Use the pH equation to calculate hydronium ion concentrations and vice versa (16-1). 5. Discuss the nature of indicators (16-2). 6. Discuss the nature of acid-base titrations (16-2). 7. Be able to complete titration problems (16-2). 8. Discuss acid-base titration curves (16-2). 2 Vocabulary acid amphoteric alkaline Arrhenius base binary acid diprotic acid triprotic acid standard solution conjugate acid end point hydronium ion pOH logarithm pH strong acid titration weak acid [H3O1+] amphiprotic base Arrhenius acid buffer oxyacid monoprotic acid polyprotic acid Ka conjugate base equivalence point indicator Kw neutralization salt strong base transition point weak base [OH1-] 3 Characteristic Properties of Acids and Bases Acids 1. 2. 3. 4. 5. Bases tastes sour conducts an electric current (electrolyte) causes certain dyes (indicators) to change color liberates hydrogen when it reacts with certain metals neutralizes basic solutions to form salts 1. 2. 3. 4. tastes bitter electrolyte causes indicators to change color feels slippery 5. neutralizes acid solutions to form salts Definition of Acids and Bases Acids and bases can be defined in many ways: Common definition . a) b) Acids are substances which dissolve in water to form acidic solutions Bases are substances which dissolve in water to form basic solutions. (A neutral solution is one that has neither acidic or basic properties.) Chemical definition a) An acid is a source of hydrogen ions (H1+) or hydronium ions (H3O1+) in solution. acid + water hydronium ion + balancing species e.g. b) HCl(aq) + H2O(l) H3O1+(aq) + Cl1-(aq) A base is a source of hydroxide ions (OH1-) in solution. base + water hydroxide ion + balancing species e.g. NaOH(s) OH1-(aq) + Na1+(aq) e.g. NH3 (aq) + H2O(l) NH41+(aq) + OH1-(aq) (A neutral solution has an equal concentration of acidic or basic ions.) Brǿnsted-Lowry definition (most practical) a) An acid is a proton donor. b) A base is a proton acceptor Acids and bases act in pairs; for an acid to donate a proton, some other species must accept a proton. Thus every reaction of this type is an acid-base reaction. 4 The reaction is an equilibrium; a species which acts as an acid on the left side of a reaction equation will act as a base on the right side of equation for the reverse reaction. These related species are called conjugate acid-base pairs. acid + base base + acid e.g. HCl(aq) + H2O(aq) H3O1+(aq) + Cl1-(aq) conjugate conjugate base acid base acid In this example the HCl and Cl- are a conjugate acid-base pair, as are H2O and H3O1+. e.g. NH3 (aq) + H2O(aq) base acid NH41+(aq) conjugate acid + OH1-(aq) conjugate base In the two examples water first acts as a base, then as an acid. Any species which can both accept and receive protons is called amphiprotic. Polyprotic and Polybasic Species Some acids are capable of donating more than one proton and some bases are capable of receiving more than one proton. These species are called polyprotic and polybasic, respectively. For example: H2SO4 - can donate 2 protons H3PO4 - can donate 3 protons Ca(OH)2 - can receive 2 protons Strength of Acids and Bases Strength is defined in terms of the degree of dissociation of the acid or base into ions. For example: HX 1+ + X 1+ + OH H BOH B 1- 1- A strong acid or base is one which completely or nearly completely dissociates into ions in solution. A weak acid or base is defined as one which only a small amount dissociates into ions in solution. If we were to look at it in terms of the equilibrium constant of dissociation, the Keq for an acid would look like this: Keq = [H1+][X1-] [HX] A strong acid would have a very high Keq since the equilibrium would favour the product. A weak acid would have a very low Keq since the equilibrium would favour the reactant. 5 Note: The equilibrium expression for an acid is referred to as a Ka; the constant for a base is a Kb. Note: A polyprotic acid would have a different Ka for each step; the value of the Ka would decrease for each step. Calculations involving acids (Ka) Strong acids are not a problem; they are considered to dissociate completely in solution: Ka = These include: HClO4 HI HBr HNO3 H2SO4 (first proton) HClO3 HCl Since these dissociate completely concentration of the hydrogen ion in solution is never in question; it is the same as the concentration of the acid in solution. Consider a 0.150 mol/L solution of HCl: HCl 0.150 mol/L H1+ + 1(0.150 mol/L) Cl11(0.150 mol/L) Weak Acids o anything with a numerical Ka value. o not all the acid dissociates, so we have to use an ICE box to determine the equilibrium [H1+]. o for example: 0.040 mol/L nitrous acid HNO2 (aq) I C 0.040 mol/L -x E 0.040 mol/L – x H1+(aq) + NO21-(aq) 0.0 +x 0.0 +x x x Ka = [H1+][NO21-] [HNO2] Ka = (x)(x) (0.040 mol/L – x) Don’t Panic!! This is not a quadratic!! Since HNO2 is a weak acid we assume that x is very small, relative to the original HNO3 concentration. We rewrite the equation as follows: Ka = (x)(x) (0.040 mol/L) Solving for x we get: x = √ (0.040 mol/L)(5.1 x 10-4) = 4.5 x 10-3 mol/L 6 Determine the [H1+] for: o 0.123 mol/L acetic acid o 0.0055 mol/L boric acid o 8.9 x 10-4 mol/L phenol Strength vs. Concentration Strength is a measure of the degree of dissociation of an acid or base. A strong acid has a high Ka value; a weak acid has a low Ka value. The same is true of strong and weak bases. Concentration is a measure how many moles of acid or base are present per litre of solution. Strength and concentration are independent terms; a strong acid can be in dilute solution, or in concentrated solution; a weak acid can be in dilute solution or in concentrated solution. e.g. Acetic acid is a weak acid; glacial acetic acid is a concentrated solution of it. Hydrochloric acid is a strong acid; most solutions of it used in class are dilute. Comparing Strength of Acids As stated earlier, the strength of an acid is measured by its Ka value. The higher the Ka, the stronger the acid. See the table in Appendix C. The table gives a ranking of acids, from strongest to weakest. According to Brǿnsted-Lowry theory, an acid is a proton donor while a base is a proton acceptor. They always work in pairs; an acid cannot donate a proton unless there is a base to accept it. If two acids are put in solution the strongest one will dominate; it will act as the acid, forcing the weaker acid to act as a base. For example: Oxalic acid and Citric acid If we take the dissociation equations from Appendix C we get HOOCCOOH H3C6H5O7 H1+ + HOOCCOO1 H1+ + H2C6H5O71- Because oxalic acid is higher on the table it is the strongest acid; it will go in the forward direction and will force citric acid to act as the base and go in the reverse direction. To solve for this reaction the citric acid equation is written in reverse and the equations are added together: HOOCCOOH H1+ + HOOCCOO11+ 1H + H2C6H5O7 H3C6H5O7 HOOCCOOH + H2C6H5O71acid base H3C6H5O7 + HOOCCOO1conjugate conjugate acid base This same procedure is done for any combination of two acids. 7 Lewis Acids and Bases A more general definition than given by Brǿnsted and Lowry, it speaks to predicting acidity and basicity for substances like ammonia, NH3 A Lewis base is an electron pair donor. A Lewis acid is an electron pair acceptor. Lewis acids and bases are best described using Lewis diagrams; for instance: H1+ [H] 1+ + + NH3 ┌ H │ │ │ H–N–H ·· │ └ ┐ │ │ │ ┘ NH41+ ┌ H │ │ │ H–N–H │ │ └ H ┐1+ │ │ │ ┘ The hydrogen ion has no valence electrons. It is looking to acquire valence electrons through a coordinate covalent bond. It associates itself with the lone pair of electrons of the nitrogen. In this bond the nitrogen donates the electron pair to the hydrogen, which is the electron pair acceptor. The hydrogen is the Lewis acid and the ammonia is the Lewis base. Another example involves boron trifluoride and ammonia: BF3 + NH3 BF3NH3 A third example involves sulfur trioxide and water, to produce sulfuric acid: SO3 + H2O H2SO4 8 Ionization of Water and Kw Acids and bases act in aqueous solution. There is a relationship between the hydronium ion and the hydroxide ion in solution. Pure water is neutral. It does have hydronium and hydroxide ions in solution which result from the dissociation of water, but they are equal in concentration. The equation for the dissociation of water is: H2O(l) H3O1+(aq) + OH1-(aq) The equilibrium constant for the dissociation of water is Keq = [H3O1+][OH1-] Since water is a liquid, it is not included in the equilibrium constant. The Keq for water, like that of acids and bases has a special notation; Kw. At most temperatures the Kw of water is 1.00 x 10-14. Since Kw is a constant, as the hydronium ion concentration increases, the hydroxide concentration decreases and vice versa. Given one concentration, the other can be calculated, using the following expression: [H3O1+][OH1-] = 1.00 x 10-14 The hydronium and hydroxide concentrations are both expressed in mol/L. In a neutral solution the concentration of both hydronium and hydroxide ions is 1.00 x 10-7 mol/L - If the hydronium ion concentration is greater than 1.00 x 10-7 mol/L the solution is acidic; - if it is less than that value, the solution will be basic (since the concentration of hydronium ions is less than 1.00 x 10-7 mol/L, the hydroxide ion concentration will be greater than 1.00 x 10-7 mol/L). 9 pH and pOH Dealing with concentrations measured in scientific notation can be difficult. To get a better handle on acidity or basicity a scale has been developed which transforms these concentrations into more manageable numbers; the pH scale. The pH can be calculated from the hydronium ion concentration: pH = - log [H3O1+] The scale is summarized below: 10 A pH of 7 represents a neutral solution ( [H3O1+] = [OH1-] ) A pH less than 7 represents an acid solution ( [H3O1+] > [OH1-] ). The lower the pH the more acidic is the solution. A pH greater than 7 represents a basic solution ( [H3O1+] < [OH1-] ). The higher the pH the more basic is the solution. pOH is similar to the pH scale, but is based on the [OH1-]. It is numbered in reverse to the pH scale; a pOH of 1 is very basic. If given a pH, it can be converted back to the hydronium ion concentration using the following formula: [H3O1+] = anti log (- pH) = 10-pH Here are some examples: Calculate the [H1+], [OH1-], pH and pOH of: i) 3.91 x 10-3 mol/L NaOH ii) 2.76 x 10-9 mol/L HNO3 iii) 4.01 x 10-4 mol/L Ba(OH)2 iv) 7.22 x 10-12 mol/L HBr v) 9.84 x 10-6 mol/L Al(OH)3 i) 3.91 x 10-3 mol/L NaOH NaOH(aq) Na1+(aq) (3.91 x 10-3 M) + OH1-(aq) 1(3.91 x 10-3 M) = 3.91 x 10-3 M [OH1-] = 3.91 x 10-3 M [H1+] = Kw [OH1-] pH = - log [H1+] = = 1.00 x 10-14 3.91 x 10-3 M - log (2.56 x 10-12 M) pOH = 14 - pH = 14 - 11.592 = 2.56 x 10-12 M = 11.592 = 2.408 11 ii) 2.76 x 10-9 mol/L HNO3 HNO3 (aq) (2.76 x 10-9 M) H1+(aq) + NO31-(aq) 1(2.76 x 10-9 M) = 2.76 x 10-9 M [H1+] = 2.76 x 10-9 M [OH1+] = Kw [H1+] = 1.00 x 10-14 2.76 x 10-9 M = 3.62 x 10-6 M pOH = - log [OH1-] = - log (3.62 x 10-6 M) = 5.441 pH = 14 - pOH = 14 - 5.441 = 8.559 iii) 4.01 x 10-4 mol/L Ba(OH)2 Ba(OH)2 (aq) Ba2+(aq) (4.01 x 10-4 M) + 2 OH1-(aq) 2(4.01 x 10-4 M) = 8.02 x 10-4 M [OH1-] = 8.02 x 10-4 M [H1+] = Kw [OH1-] pH = - log [H1+] = 1.00 x 10-14 8.02 x 10-4 M = - log (1.25 x 10-11 M) pOH = 14 - pH = 14 - 10.904 = 1.25 x 10-11 M = 3.096 = 10.904 12 iv) 7.22 x 10-12 mol/L HBr HBr (aq) (7.22 x 10-12 M) H1+(aq) + Br1-(aq) 1(7.22 x 10-12 M) = 7.22 x 10-12 M [H1+] = 7.22 x 10-12 M [OH1+] = Kw [H1+] = 1.00 x 10-14 7.22 x 10-12 M pOH = - log [OH1-] = - log (1.38 x 10-3 M) = 1.38 x 10-3 M = 2.858 pH = 14 - pOH = 14 - 2.858 = 11.141 v) 9.84 x 10-6 mol/L Al(OH)3 Al(OH)3 (aq) Al3+(aq) + (9.84 x 10-6 M) 3 OH1-(aq) 3(9.84 x 10-6 M) = 2.95 x 10-5 M [OH1-] = 2.95 x 10-5 M [H1+] = Kw [OH1-] pH = - log [H1+] = 1.00 x 10-14 2.95 x 10-5 M = 3.39 x 10-10 M = - log (3.39 x 10-10 M) = pOH = 14 - pH = 14 - 9.470 = 4.530 9.470 13 Titration The concentration of H1+ or OH1- is very important, especially in products manufactured for human use. These concentrations are measured using a method called titration. This involves neutralizing a sample of an unknown acid solution with a known concentration of base (or vice-versa). At the neutralization point the moles of acid equals the moles of base c = which is altered to n V n = cV The moles of base added is thus equal to: nb = cbVb and the moles of acid originally in solution is equal to na = caVa Since at the point of neutralization the moles of acid and base are equal, the following formula is arrived at: caVa = cbVb Note the similarity between this equation and the dilution equation used earlier in the year. Example Calculations: What is the concentration of a base if 50.0 mL of the base is neutralized by 16.1 mL of a 0.100 mol/L solution of acid ? cb vb ca va = = = = ? 50.0 mL 0.100 mol/L 16.1 mL cava = cbvb cb = cava vb cb = (0.100 mol/L)(16.1 mL) (50.0 mL) cb = 0.0322 mol/L 14 Calculate the concentration of HCl if 100. mL of an HCl solution is neutralized by 56.3 mL of a 0.500 mol/L solution of NaOH ca va cb vb = = = = ? 100. mL 0.500 mol/L 56.3 mL ca = ca = cbvb va (0.500 mol/L)(56.3 mL) (100. mL) ca = 0.282 mol/L HCl Indicators How does one know when the neutralization point has been reached when titrating? There are two ways. By far the most popular method today is measuring the pH electronically using pH electrodes and computerized titrators. If a titration is followed using a pH meter the results can be graphed and a titration curve results: This curve represents the addition of a base to an acidic solution. The pH changes very slowly until the equivalence point is approached, where the moles of acid equals the moles of base and the solution is neutral. The the pH changes very rapidly, often with the addtion of just one or two drops of additional base. It is by this rapid change in pH that the equivalence point may be recognized. The volume of base needed to reach the equivalence point can be determined by the graph (Vb). If the concentration of the base is known (Cb) and the original volume of acid is known (Va) then the titration equation can be used to get the concentration of the acid (C a) 15 If the titration is done with a polyprotic acid, like phosphoric acid, the following titration curve results: The curve has three stages representing the equivalence point for each of the three hydrogen ions in H 3PO4. An older method of titration uses indicators. These are chemicals which are pH sensitive. They undergo a colour change as the pH shifts from acid to basic conditions. The neutralization point is detected when the solution changes colour. The following table lists some indicators along with their colour change correlated to the pH range: Indicators are the method of choice in student labs because they are cheap and easy to use. They are also used in the field, where instrumentation is limited. Indicators are used not only in acid-base reactions, but in a broad range of chemical reactions, including redox reactions (next chapter). 16 Applications of Acid Base Chemistry Buffers Living organisms can live in only a very narrow pH range. This applies both inside the organism and outside. If the internal pH of an organism drops much beyond 7.4 sickness and death can result through acidosis. This could happen easily if the CO2 produced as a result of respiration dissolved in the bloodstream to form carbonic acid. This could drop the pH to as low as 6.4. Fortunately there are other weak acids and bases in our systems which act to reduce, or buffer, the acid effects. These compounds prevent the dissociation of the acid which allows it to exist harmlessly in our systems until it can diffuse out of our lungs as CO2. These buffer systems include proteins in plants and animals as well as the phosphates, carbonate and ammonium salts found in the earth. Buffers are also used in consumer and industrial products to ensure their pH range is maintained while the product is shipped and consumed. Acid Rain Whenever coal or petroleum products are burned there are byproducts in the form of nitrogen oxides (NO x) and sulphur oxides (SOx). These oxides react with oxygen and water in the air to form nitric acid (HNO 3) and sulphuric acid (H2SO4). If the gases are released at ground level by cars and trucks they cause corrosion of metals and decay of concrete and stone. If the gases are released in smoke stacks they can travel large distances before they fall in the form of acid precipitation. If acid rain falls on unbuffered soils and lakes the pH can fall rapidly affecting the health of the living organisms and releasing toxic metals into the ground water. This can lead to wholesale death of lakes and forests. If the gases are treated with lime (CaO) in the smokestack the SO x gases can be precipitated. Catalytic converters on car exhaust can convert the NOx to harmless N2.