mixture electron
advertisement

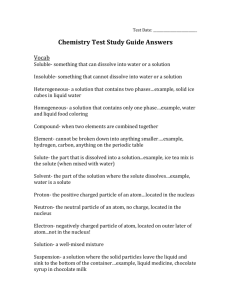
Name: _______________________________________ Chemistry Test Review Guide Vocabulary Atom Atomic mass Atomic number Atomic structure Chemical formula Chemical property Column Compound Conductivity Conservation of mass Density Electron Element Group Halogens Isotope Malleability Metalloids Metals Mixture Molecule Neutron Nonmetals Non-reactivity Period Physical property Precipitate Proton Reactivity Solubility Solubility Valence electrons Volume 1. Explain how the properties of elements are related to the properties of the compounds that the elements make. The properties of elements are different from the elements they create. Take water, for example. Water is made of hydrogen and oxygen – two very explosive elements. Water, on the other hand is the opposite; water puts out fires. 2. Explain how the Periodic Table is organized. Who originally organized it? The Periodic Table is organized by increasing atomic number. The columns on the P.T. are called families and the elements in each family tend to have properties that are similar. Dmitri Mendeleev first organized the Periodic Table. 3. What are the families on the Periodic Table? What is important about each family? What are the characteristics of each family? The families on the Periodic Table are the columns. The first column – the Alkali Metals are very reactive. The last column – the Noble Gases – are not reactive at all. 4. Draw and label a Fluorine atom. Protons: 9 Neutrons: 10 Electrons: 9 5. 1. 2. 3. 6. List the four signs that a chemical change has occurred. Color change Temperature change Gas (or bubbles) form A precipitate (a solid) forms 7. What is the difference between a physical change and a chemical change? A physical change is a change where matter changes form or appearance. Examples of physical changes include crushing a can, bending a wire, water evaporating, or tearing a paper. A chemical change is when a new substance is formed. There are four signs that a chemical change has occurred. Examples of chemical changes include rusting metal, tarnishing silver, or burning wood. 8. Explain conservation of mass. If I start with 100 grams of reactants, how many grams of products will I produce? Conservation of mass is the idea that you will have the same amount of matter before a reaction as at the end of a reaction. If you start with 100 grams of reactants, you will end with 100 grams of product. 9. What is a mixture? What is the difference between a homogeneous mixture and a heterogeneous mixture? A solution is which type of mixture and why? A mixture is two or more types of matter mixed together, but not chemically combined. A mixture, unlike a compound, can be physically split into the matter that it is made up of. A homogenous mixture is a mixture where you cannot visibly see the different pieces of matter that make up it (like Kool-aid or brass). A heterogenous mixture is a mixture where the individual pieces of matter are visible (like chocolate chip cookies or a salad). A solution is a homogenous mixture because the substances in the solution are mixed to where you cannot see the different substances. 10. Explain the differences between protons, electrons, and neutrons. Be sure to mention where they are located in an atom, charge, and mass. Charge Mass Location Proton + 1 Nucleus Electron 0 Electron orbital Neutron 0 1 Nucleus 11. What do valence electrons have to do with reactivity? Valence electrons are the electrons on the outermost orbital of the atom. When an atom has one or two valance electrons, the element tends to be more reactive. When an atom has a full outer orbital, the atom is not reactive at all. 12. What is the difference between atomic mass and atomic number? How could you use these to find the number of protons, neutrons, and electrons in an atom? Atomic mass is the number of protons plus the number of neutrons. The atomic number is the number of protons. Atomic number = number of protons Number of protons = number of electrons Atomic mass – atomic number = number of neutrons 13. What is the difference between a solute, solvent, and solution? A solution is made up of a solute and solvent. The solvent is the substance that there is more of and dissolves the solute. In salt water, the water is the solvent and the salt is the solute. In air, nitrogen gas is the solvent (because there’s more nitrogen gas) and carbon dioxide and other gases are the solutes. 14. How does a mixture differ from a compound? A mixture can be physically broken down into the different substances that make it. A compound cannot be broken down by physical means. 15. 16. Balance the following equations. a. 2 Fe + 3Cl2 2 FeCl3 5O2 b. C3H8 + c. C6H4Cl2 + 4H2O + 4O2 3CO2 6 CO + 2 H2O+ Cl2 In #14, put a circle around the reactants and a rectangle around the products.