Fe + CuCl → FeCl
advertisement
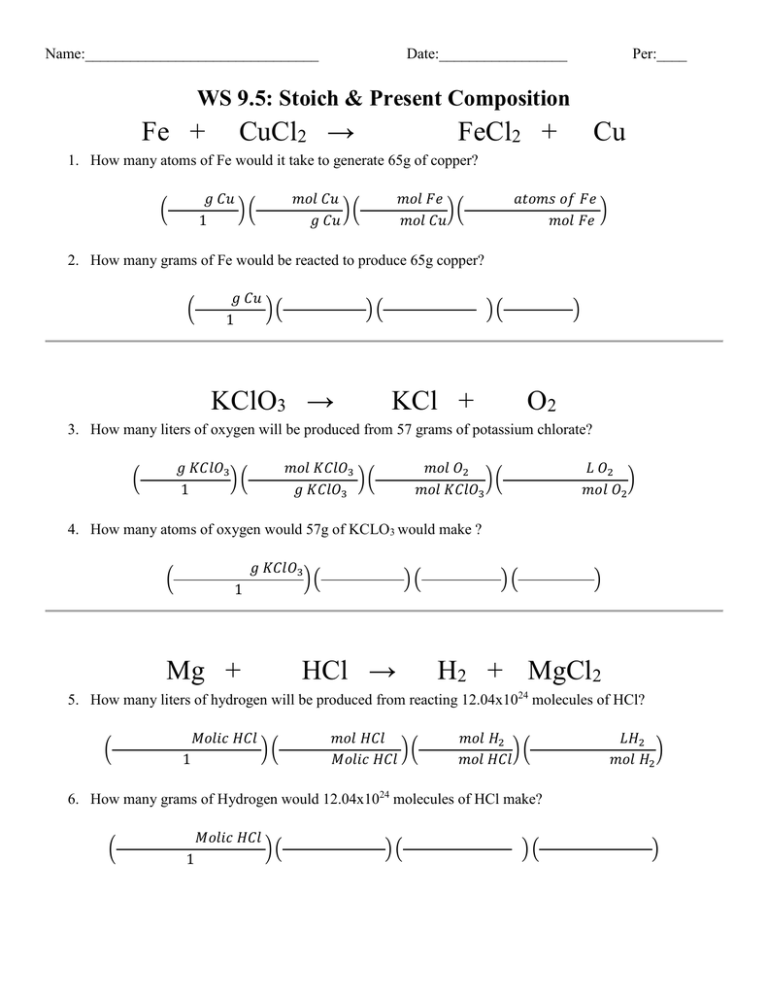
Name:_______________________________ Date:_________________ Per:____ WS 9.5: Stoich & Present Composition Fe + CuCl2 → FeCl2 + Cu 1. How many atoms of Fe would it take to generate 65g of copper? 𝑔 𝐶𝑢 )( 1 ( 𝑚𝑜𝑙 𝐶𝑢 )( 𝑔 𝐶𝑢 𝑚𝑜𝑙 𝐹𝑒 )( 𝑚𝑜𝑙 𝐶𝑢 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝐹𝑒 ) 𝑚𝑜𝑙 𝐹𝑒 2. How many grams of Fe would be reacted to produce 65g copper? ( 𝑔 𝐶𝑢 )( 1 )( KClO3 → )( KCl + ) O2 3. How many liters of oxygen will be produced from 57 grams of potassium chlorate? 𝑔 𝐾𝐶𝑙𝑂3 )( 1 ( 𝑚𝑜𝑙 𝐾𝐶𝑙𝑂3 )( 𝑔 𝐾𝐶𝑙𝑂3 𝑚𝑜𝑙 𝑂2 )( 𝑚𝑜𝑙 𝐾𝐶𝑙𝑂3 𝐿 𝑂2 ) 𝑚𝑜𝑙 𝑂2 4. How many atoms of oxygen would 57g of KCLO3 would make ? 𝑔 𝐾𝐶𝑙𝑂3 ( 1 )( )( HCl → Mg + )( ) H2 + MgCl2 5. How many liters of hydrogen will be produced from reacting 12.04x1024 molecules of HCl? ( 𝑀𝑜𝑙𝑖𝑐 𝐻𝐶𝑙 1 )( 𝑚𝑜𝑙 𝐻𝐶𝑙 )( 𝑀𝑜𝑙𝑖𝑐 𝐻𝐶𝑙 𝑚𝑜𝑙 𝐻2 )( 𝑚𝑜𝑙 𝐻𝐶𝑙 𝐿𝐻2 ) 𝑚𝑜𝑙 𝐻2 6. How many grams of Hydrogen would 12.04x1024 molecules of HCl make? ( 𝑀𝑜𝑙𝑖𝑐 𝐻𝐶𝑙 1 )( )( )( ) C5H10 + O2 → CO2 + H2 O 7. How many grams of H20 will be generated from 140 grams of pentene? ( 𝑔 𝐶𝑢 )( 1 𝑚𝑜𝑙 𝐶𝑢 )( 𝑔 𝐶𝑢 𝑚𝑜𝑙 𝐹𝑒 )( 𝑚𝑜𝑙 𝐶𝑢 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝐹𝑒 ) 𝑚𝑜𝑙 𝐹𝑒 8. 140g of pentene combusting would make how many L of water vaper? 𝑔 𝐶𝑢 )( 1 ( )( CO2 → )( C + ) O2 9. If 44.8 L of O2 are produced from the decomposition of carbon dioxide, how many liters of carbon dioxide decomposed? ( 𝑔 𝐶𝑢 )( 1 𝑚𝑜𝑙 𝐶𝑢 )( 𝑔 𝐶𝑢 𝑚𝑜𝑙 𝐹𝑒 )( 𝑚𝑜𝑙 𝐶𝑢 𝑎𝑡𝑜𝑚𝑠 𝑜𝑓 𝐹𝑒 ) 𝑚𝑜𝑙 𝐹𝑒 10. If 44.8 L of O2 are produced from the decomposition of carbon dioxide, how many liters of carbon dioxide decomposed? ( 𝑔 𝐶𝑢 )( 1 )( )( ) 11. A 3.6 sample is found to contain only carbon and iron. Given that the sample contains 1.68 g of iron, calculate the percentage composition of the compound. 12. Find the percentage composition of a compound that contains 4.8 g of hydrogen, 19.4 g of carbon, , and 25.8 g of sulfur in a 50.0 g sample of the compound. 13. A sample of an unknown compound with a mass of 16.5g has the following composition: 86.2% nitrogen and the rest 13.8% chlorine. When this compound is decomposed into its elements, what mass of each element would be recovered?





